Humanized recombinant uricase and mutant thereof
A uricase and mutant technology, applied in the field of DNA recombination technology and medicine, can solve the problems of lack of selection pressure, reduction of enzyme immunogenicity, inability to use long-term treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0046] Example 1 Recombinant expression of human dog chimeric uricase protein UHC in Escherichia coli
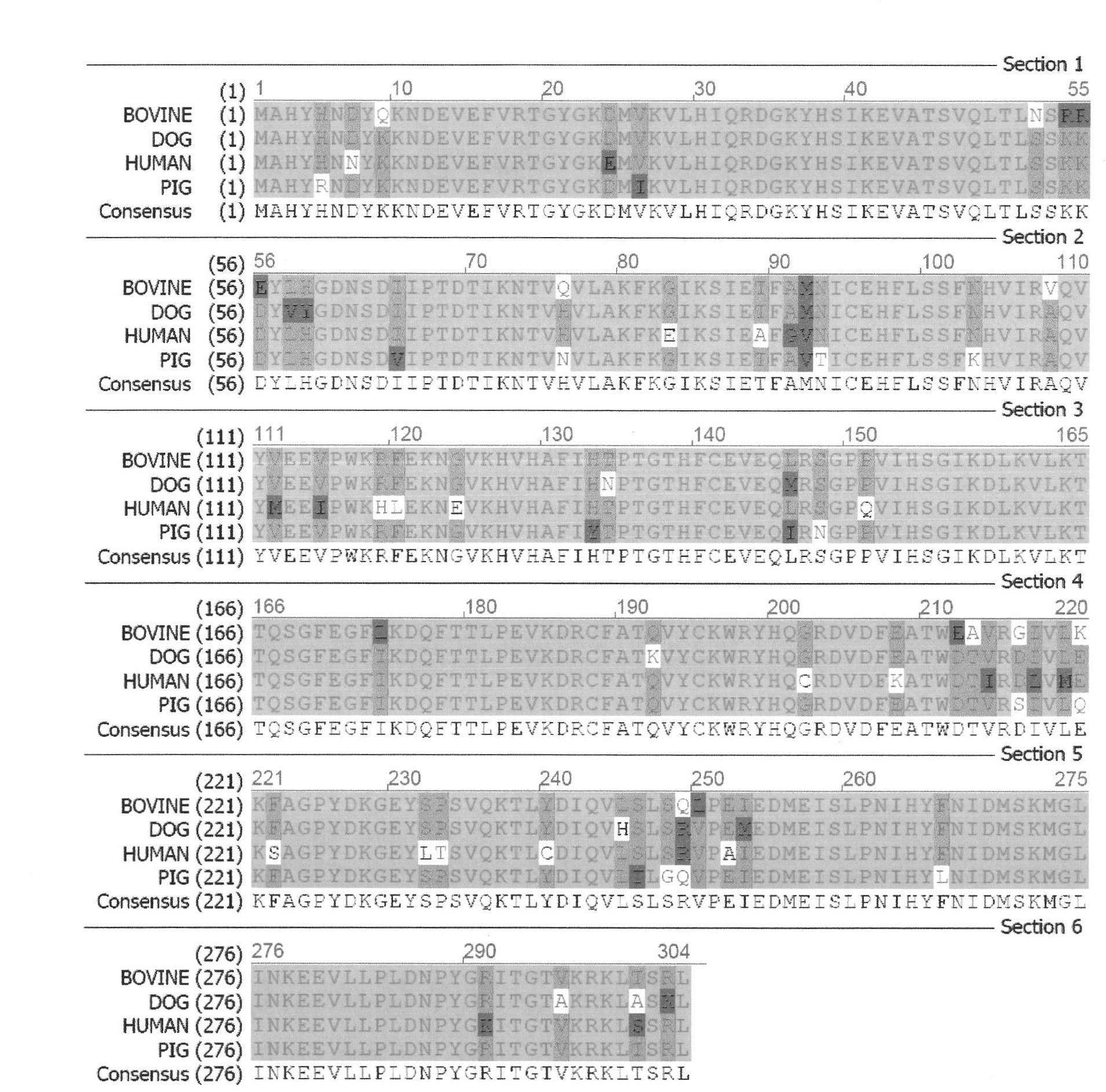
[0047] In this example, the first 240 amino acids at the N-terminal of the involved chimeric protein are the uricase sequence of dog origin, and the 241-304th amino acid sequence is the amino acid sequence of uricase of human origin. According to the above amino acid sequence and according to the codon preferred by Escherichia coli, Bao Biology (Dalian) Co., Ltd. was entrusted to carry out the whole gene synthesis. The nucleotide sequences are (Seq ID No: 10): CAT ATG GCC CAT TAT CAT AAT GAT TAT AAA AAA AAT GAT GAA GTT GAA TTT GTT CGT ACC GGT TAT GGTAAA GAT ATG GTT AAA GTT CTG CAT ATT CAG CGT GAT GGT AAA TAT CAT TCT ATT AAA GAA GTT GCC ACCTCT GTT CAG CTG ACC CTG TCT TCT AAA AAA GAT TAT GTT TAT GGT GAT AAT TCT GAT ATT ATT CCA ACCGAT ACC ATT AAA AAT ACC GTT CAT GTT CTG GCC AAA TTT AAA GGT ATT AAA TCT ATT GAA ACC TTT GCCATG AAT ATT TGT GAA CAT TTT CTG TCT TCT TTT AAT CAT GTT AT...
example 2
[0052] Example 2 Human dog chimeric uricase protein UHC mutant DNA construction and recombinant expression in Escherichia coli
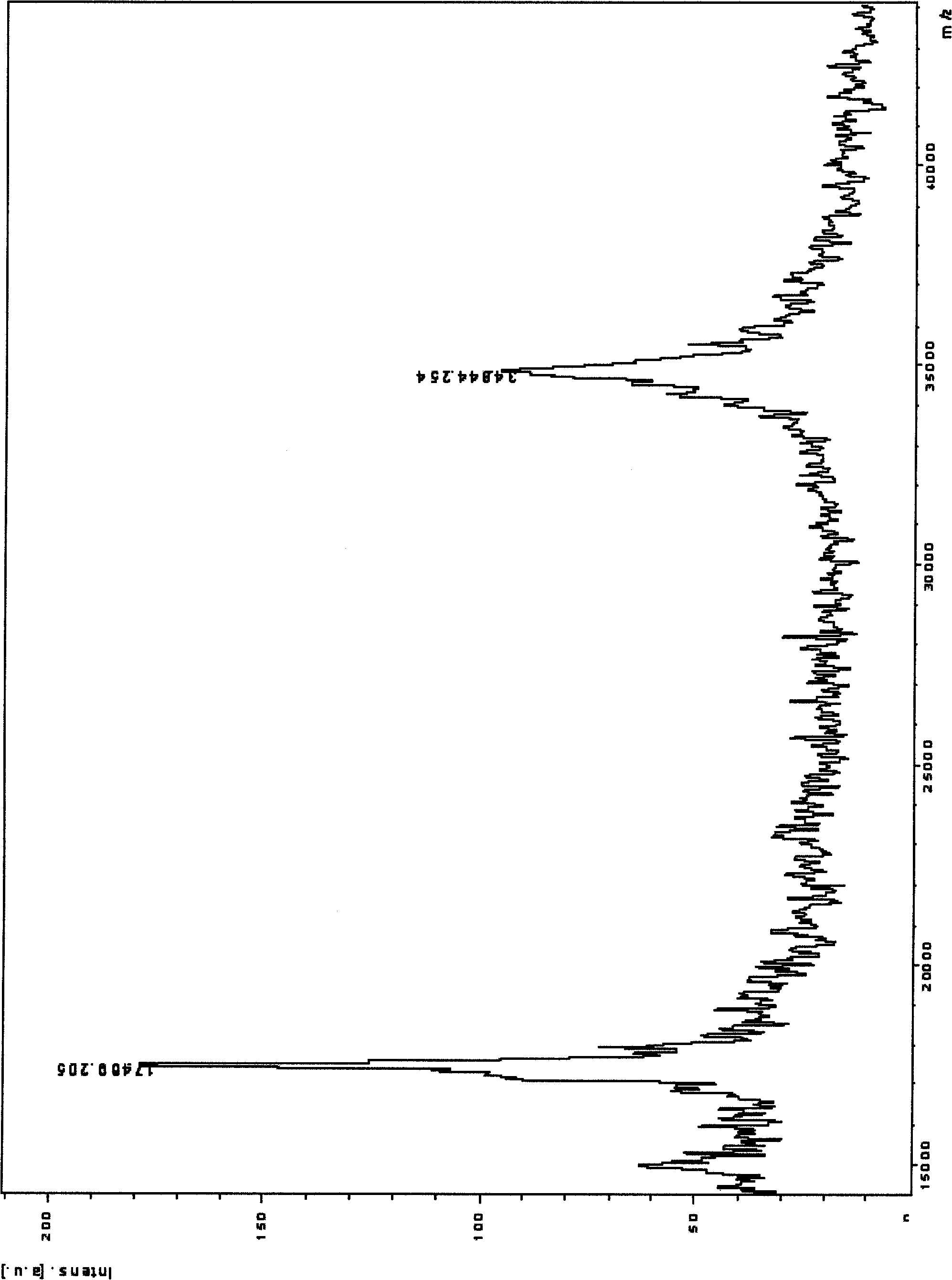
[0053] Using the UHC protein DNA sequence as the original template, the DNA containing the target mutation was mutated by the staggered extension PCR method (attached image 3 ). The following is an example of preparing S246T-S248G-R249Q and S246T-S248G-R249Q-F266L:
[0054] Primer number
dna sequence
Primer 1 (Seq ID No: 11)
5′CACGACATATGGCCCATTATCATA 3′
Primer 2 (Seq ID No: 12)
5′ GGATCCTTATCACAGACGAGAA 3′
Primer 3 (Seq ID No: 13)
5′ACCCTGGGTCAGGTTCCAGAAATTGAAGATATGGAAATT 3′
Primer 4 (Seq ID No: 14)
5′TTCTGGAACCTGACCCAGGGTCAGAACCTGAATATCATA3′
Primer 5 (Seq ID No: 15)
5′TTCATTATCTGAATATTGATATGTCTAAAAA 3′
Primer 6 (Seq ID No: 16)
5′ATCAATATTCAGATAATGAATATTGGCAG 3′
[0055] Prepare UHC 246 / 248 / 249 : first-stage PCR: the template sequence is the whole gene synthesis...
example 3U
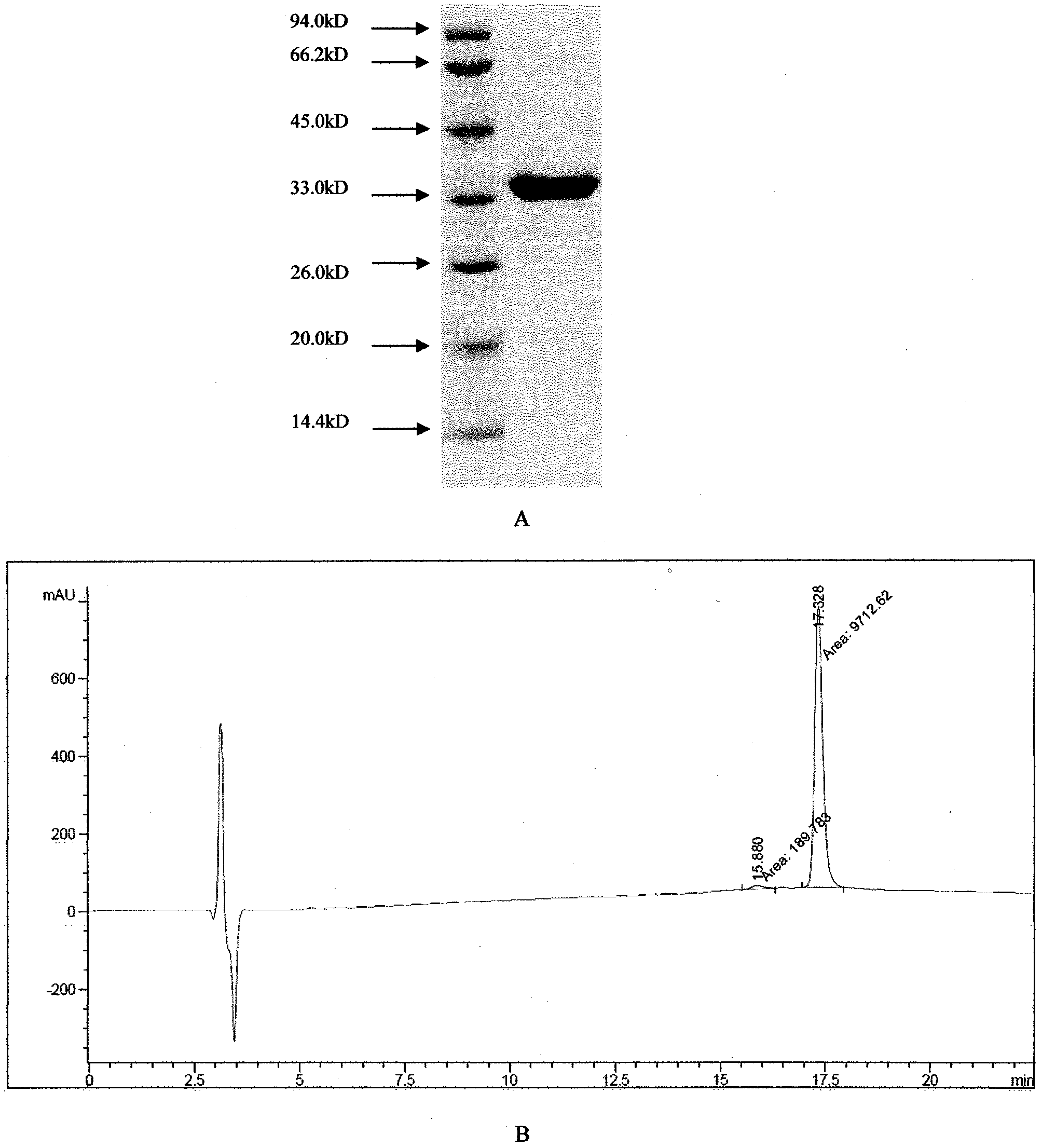
[0058] Example 3UHC chimeric protein and mutant protein Escherichia coli expression product purification
[0059] Take 50g of bacterial precipitates and add 500ml of bacteriostasis solution, the pH of the bacteriostasis solution is 8.2, containing 25mM Tris-HCl, 0.1mg / ml lysozyme, stir at 37°C for 2 hours, and ultrasonically destruct the above bacteria solution (500W 4S, interval 6S, 30 times); centrifuge to discard the supernatant, precipitate with 2L pH 10.20.1M Na 2 CO 3 Dissolve and stir overnight at room temperature; discard the precipitate by centrifugation, add 15% saturated ammonium sulfate to the supernatant, and precipitate at 4 degrees for 2 hours; discard the supernatant by centrifugation, and use 2L pH 10.20.1M Na 2 CO 3 Dissolve and stir at room temperature overnight; centrifuge to discard the precipitate, and the supernatant is purified by QAE agarose anion exchange chromatography column (GE). 2 CO 3 ) was eluted, and the target protein was eluted at 0.3M Na...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com