Construction of recombinant strain capable of producing arginine deiminase and directional modification method thereof
A technology of arginine deiminase and amino acid, applied in the direction of microorganism-based methods, botany equipment and methods, biochemical equipment and methods, etc., can solve problems such as limiting the application prospects of recombinant ADI enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Construction of recombinant ADI
[0024] According to the gene sequence of the ADI, design primers:
[0025] F: 5'-GCT CATATG TCCGCTGAAAAACAGAAGTACG-3’ ( Nde I)
[0026] R: 5'-AT CTCGAG TTAGTAGTTGATCGGGTCGCGCA -3' ( xho I)
[0027] Introduce upstream and downstream separately Nde I and xho I enzyme cutting site (underlined), the primers used in the present invention were all synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd.
[0028] Using Pseudomonas mutans cells as a template, the ADI gene fragment was amplified by PCR, and the reaction system was 50 μL, as shown in Table 1:
[0029] Table 1 PCR amplification reaction system of ADI gene fragment
[0030] 10×Ex Taq Buffer 5 μL 2.5 mM dNTPs 4 μL F-Primer (20μM) 1 μL R-Primer (20μM) 1 μL bacteria - Ex Taq DNA Polymerase (5 U / μL) 0.5 μL ddH2O 38.5 μL
[0031] Reaction program: Denaturation at 94 °C for 10 min; rea...
Embodiment 3
[0051] Example 3 Screening of ADI mutant library
[0052] A single colony in the ADI mutant library was transferred to an LB / Kan plate containing IPTG (final concentration 0.2 mmol / L), and cultured at 30°C until a single colony grew.
[0053] According to the reaction that ADI catalyzes arginine to produce citrulline and ammonia, a 96-well plate screening method for mutants with ADI activity was designed. Specific operation: Take a 96-well plate, first add 50 μl of 0.2mol / L phosphate buffer solution (pH 7.4) to each well, pick a single colony induced by IPTG and mix it in the well (the sample without bacteria is used as a control), Then add 50 μl of 1 mmol / L L-arginine hydrochloride, 0.2 mol / L phosphate buffer (pH 7.4), react at 37°C for 15 min, add 90 μl of mixed acid to terminate the reaction, and then add 30 μl of diacetylmonoxime-sulfur Amidurea solution, mix well, react at 37°C for 2 hours, measure OD 530 . The reaction solution in the well where the strain with ADI ac...
Embodiment 4
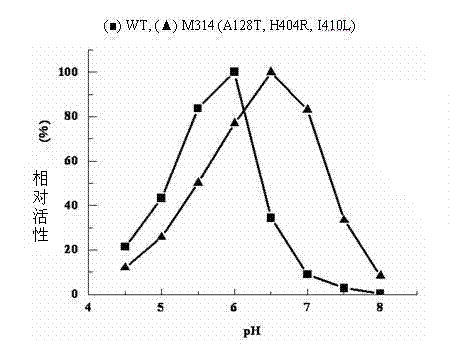
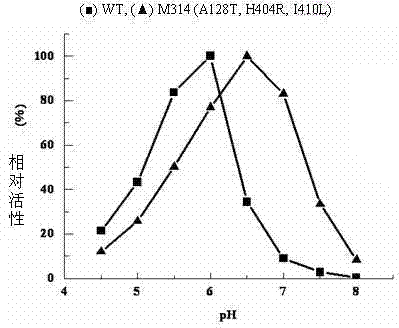
[0054] Example 4 ADI mutant strain M314
[0055] A single colony of the ADI mutant strain M314 was inserted into LB / Kan liquid medium and cultured overnight at 37°C and 200r / min. Bacteria were collected, plasmids were extracted, and purified. The plasmid was sequenced by Shanghai Sangon Bioengineering Technology Service Co., Ltd. See SEQ ID NO: 1 in the sequence table. It was determined that M314 carried three mutation sites, namely A128T, H404R and I410L.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com