Quality control method for gastric condition-regulating pill as traditional Chinese preparation
A quality control method and a technology of traditional Chinese medicine preparations, which are applied in the directions of medical formulas, measuring devices, drug combinations, etc., can solve the problems of identification items, identification means, content determination methods, etc., and achieve the effect of improving quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
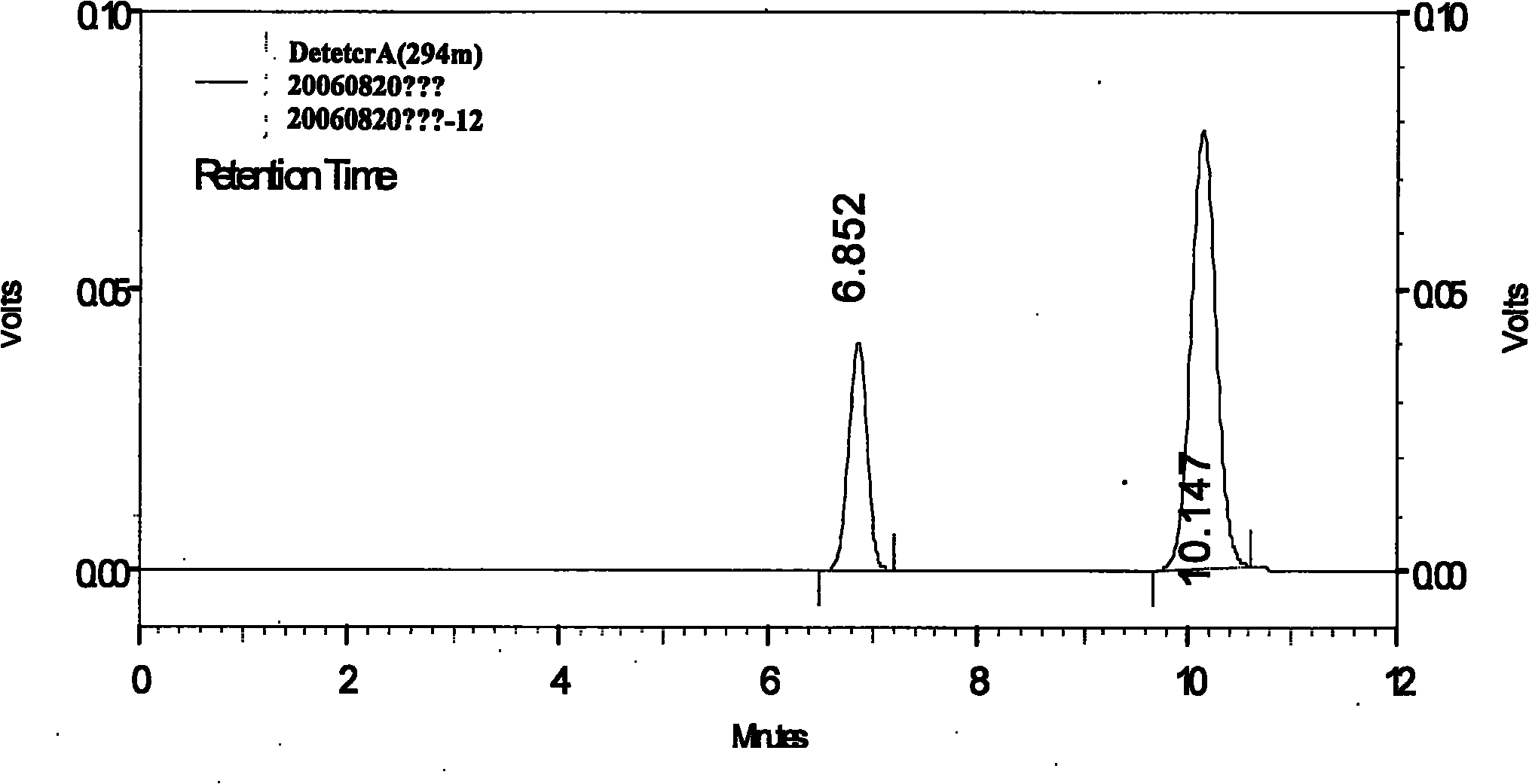
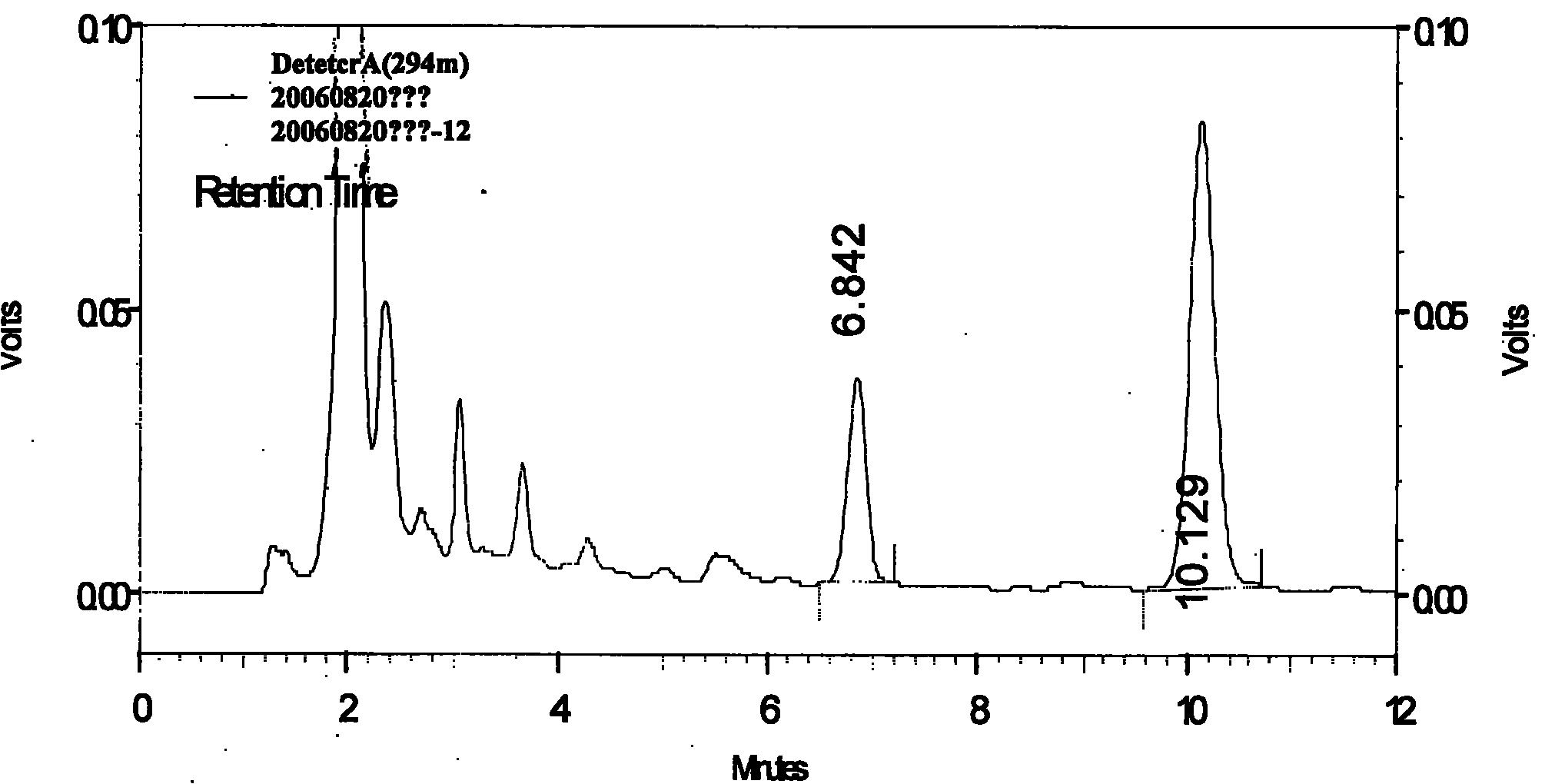
[0064] Preparation of Reference Substance Solution Take appropriate amount of magnolol reference substance and honokiol reference substance, weigh them accurately, add methanol to make a solution containing 130 μg of magnolol and 45 μg of honokiol per 1 ml.
[0065] Preparation of the test solution Take an appropriate amount of this product, grind it into a fine powder, take 0.5g of the powder, accurately weigh it, accurately add 25ml of methanol, weigh it, treat it ultrasonically for 30 minutes, let it cool, weigh it again, and make up for it with methanol Subtract the lost weight, shake well, filter, take the continued filtrate, that is.
[0066] Determination method Draw the above-mentioned reference substance solution and 5 μ l of the test solution respectively, inject it into the liquid chromatograph, measure it, and obtain it. Each 1g of this product contains magnolol and magnolol (C 18 h 18 o 2 ) and honokiol (C 18 h 18 o 2 ) shall not be less than 1.6mg.
[0067...
Embodiment 2
[0069] Example 2 Tiaoweidan Quality Standard Drafting Instructions:
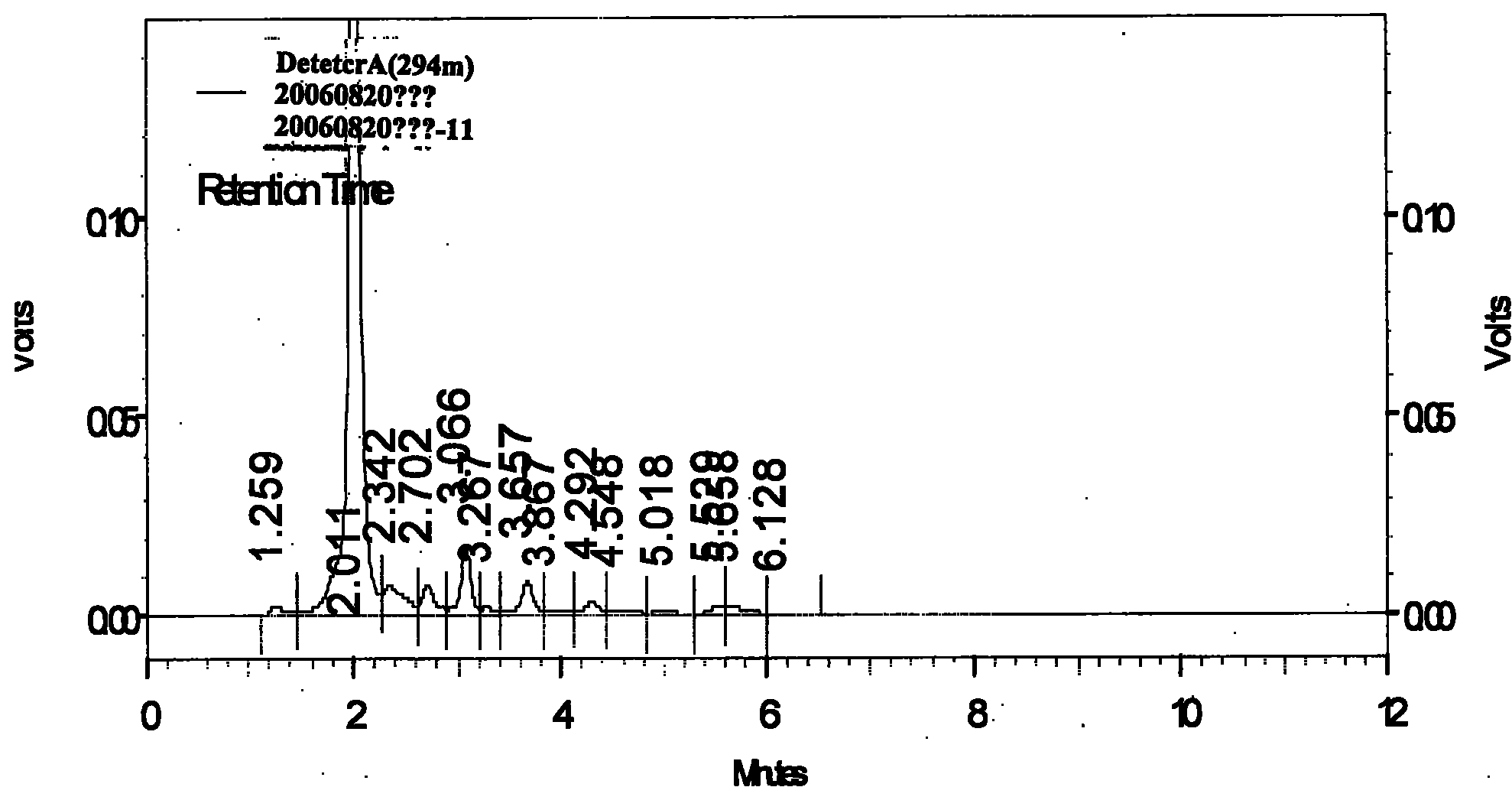
[0070] Identification:
[0071] 1. Instruments and reagents
[0072] High performance liquid chromatography: Shimadzu LC-2010A HT
[0073] Ultrasonic instrument: KUDOS SK8200LH (Shanghai Kedao Ultrasonic Instrument Co., Ltd.);
[0074] Electronic balance: Sartorius CP225D / CP224S (Beijing Sartorius Instrument System Co., Ltd.);
[0075] Silica gel G thin-layer board, silica gel H thin-layer board, high-efficiency silica gel G thin-layer board (Qingdao Ocean Chemical Factory Branch);
[0076] Reference substance: dehydrocresylide (batch number: 1525-200102)
[0077] Synephrine (Lot No.: 110727-200105)
[0078] The above reference substances were all purchased from China National Institute for the Control of Pharmaceutical and Biological Products;
[0079] The reagents used are analytically pure, and the water is redistilled deionized water;
[0080] Tiao Wei Dan (batch number: 060301, 060302, 0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com