Metal utilization in supported, metal-containing catalysts
A catalyst, oxidation catalyst technology, applied in the field of guiding and/or controlling metal deposition to the surface of porous substrates, treating porous substrates to provide treated substrates, and preparing catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
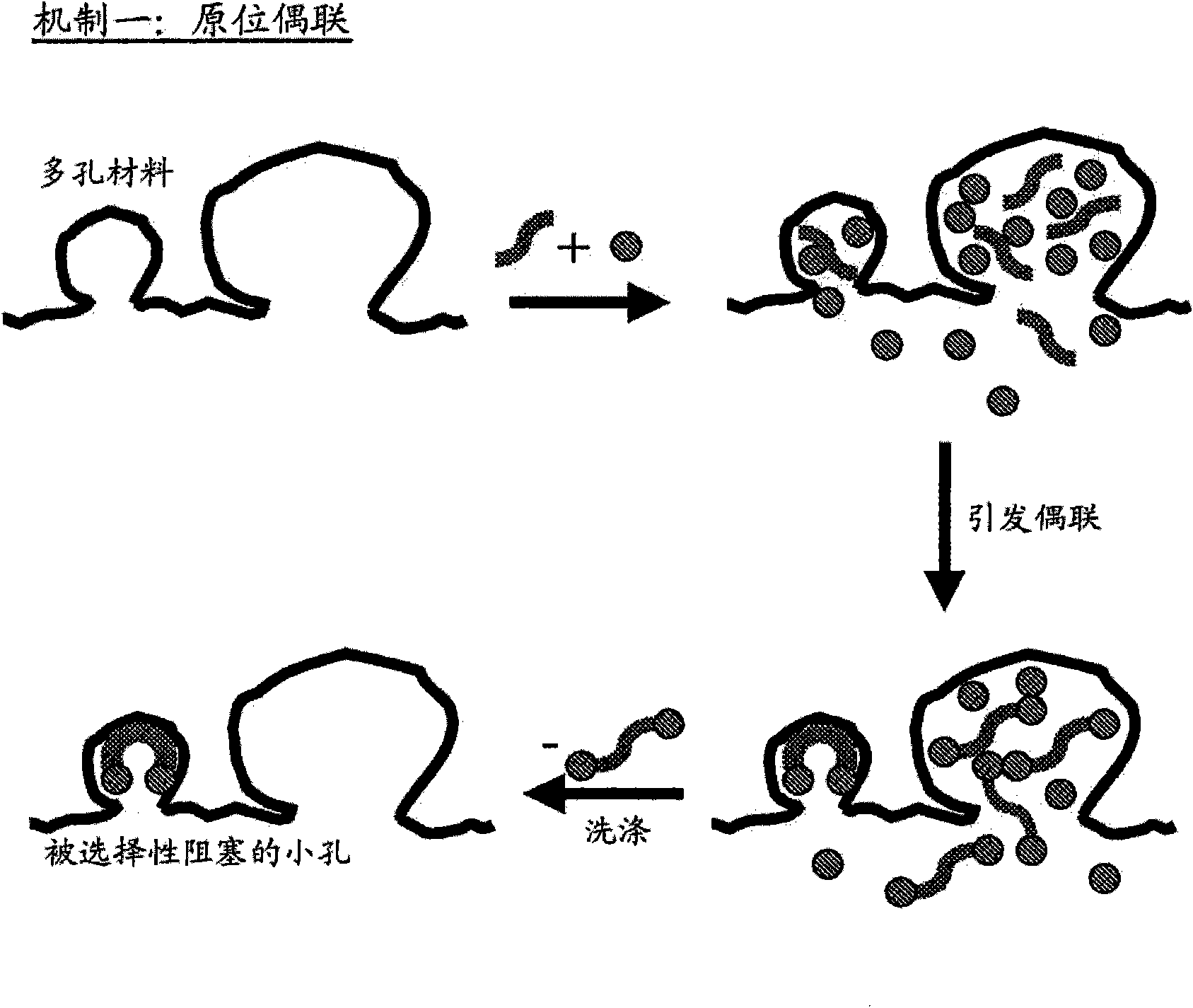
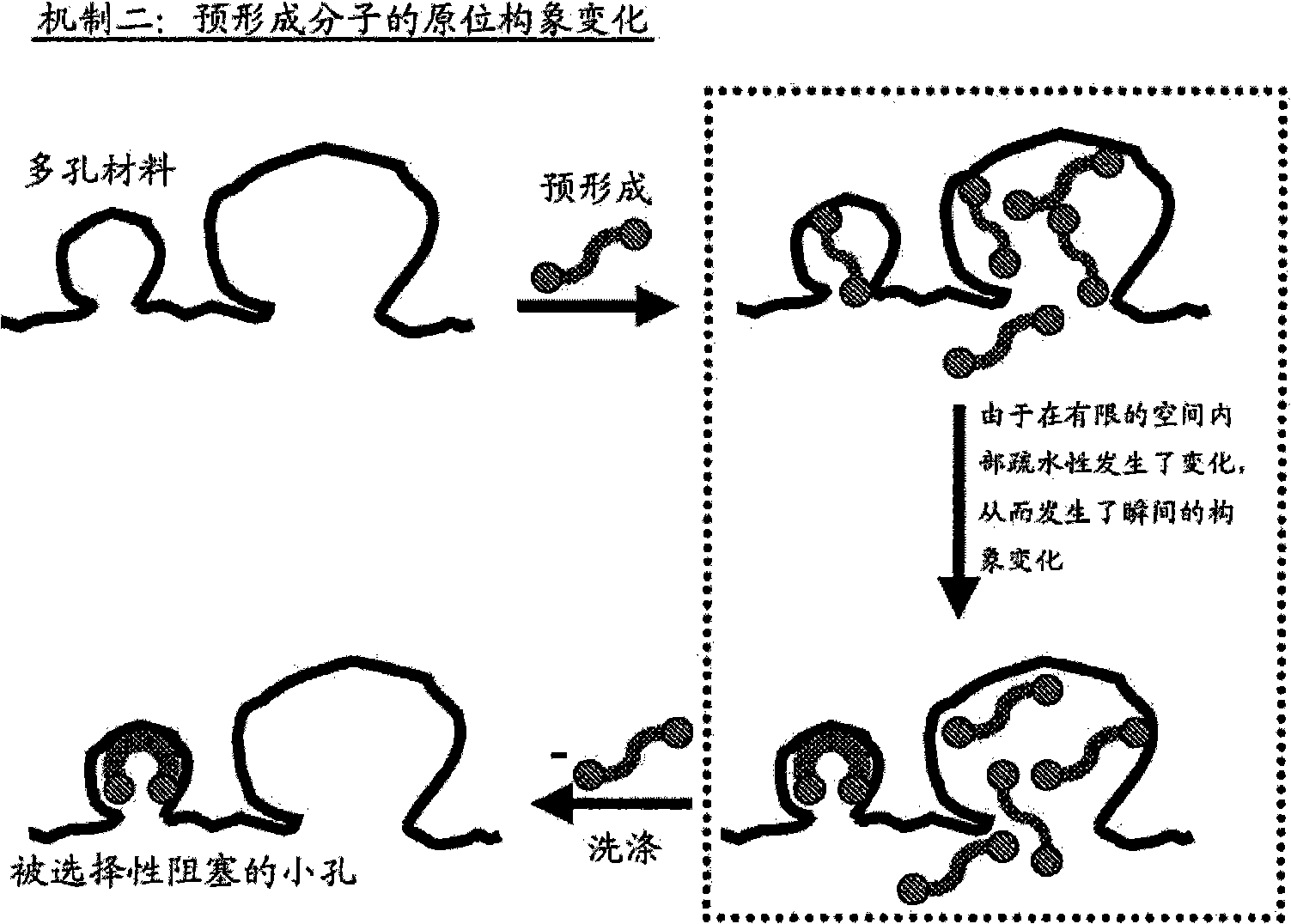
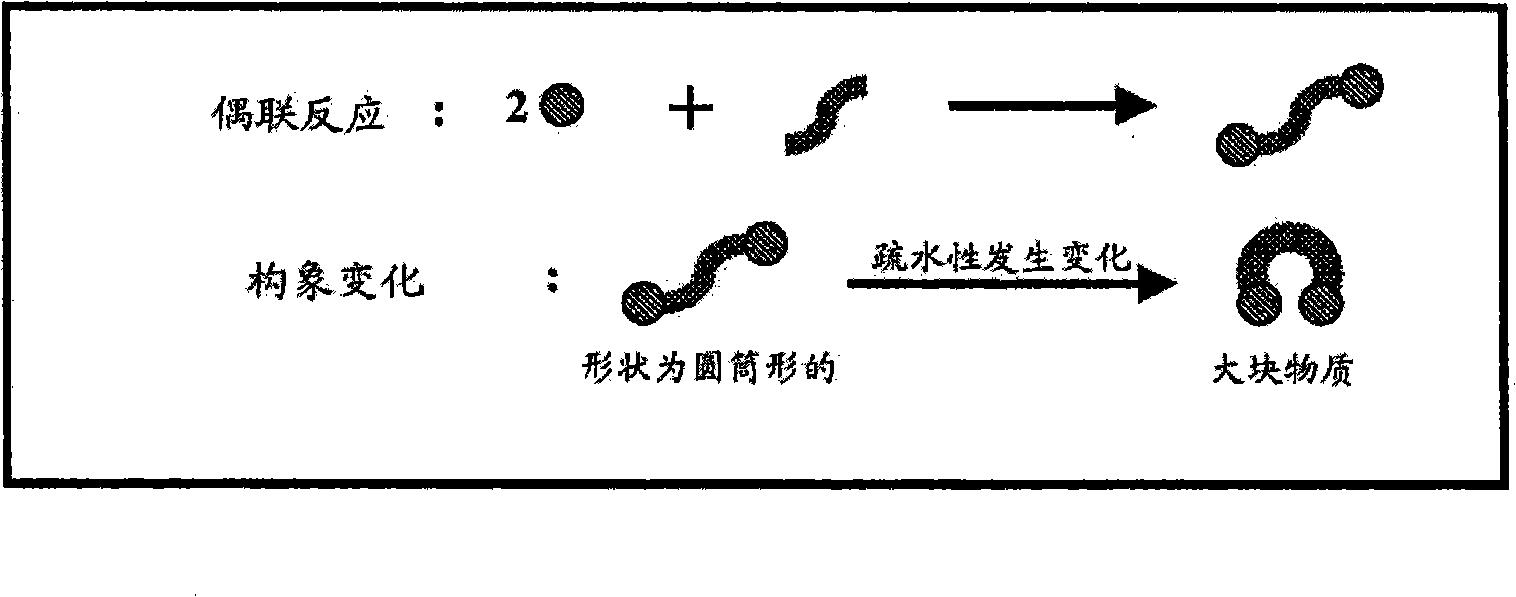
[0142] The preparation of catalysts is described herein, thereby providing improvements in the use of metals in supported metal-containing catalysts. In general, various embodiments of the invention include controlling and / or directing metal deposition onto the surface of a porous substrate. Controlled or directed metal deposition can be employed to address one or more of the problems associated with the preparation of conventional supported metal-containing catalysts.
[0143] For example, one potential disadvantage associated with conventional platinum-on-carbon catalysts is that relatively small platinum-containing particles are prone to leaching during liquid-phase catalytic oxidation reactions compared to larger metal-containing particles. Excessive leaching of metal particles results in metal loss and manifests as inefficient metal utilization. Furthermore, in the case of PMIDA oxidation, it is believed that these relatively small platinum-containing crystallites contri...
Embodiment 1
[0360] Three carbon supports were treated to determine the potency of candidate pore-blocking compounds. Carrier A has a total Langmuir surface area of approximately 1500 m 2 / g (including the total surface area of micropores is about 1279m 2 / g and the total macropore surface area is about 231m 2 / g). Carrier B has a total Langmuir surface area of approximately 2700 m 2 / g (including the total surface area of micropores is about 1987m 2 / g and the total macropore surface area is about 723m 2 / g). Carrier C has a total Langmuir surface area of about 1100 m 2 / g (including the total surface area of micropores is about 876m 2 / g and the total macropore surface area is about 332m 2 / g).
[0361] The candidate pore-blocking compound is 1,4-cyclohexanedione, ethylene glycol, and the diketal product of a coupling reaction between 1,4-cyclohexanedione and ethylene glycol (that is, 1, 4-Cyclohexanedione bis(ethylene glycol ketal)).
[0362] A sample of support (...
Embodiment 2
[0371] Carbons A, B and C (30 g) described in Example 1 were contacted with a solution of 1,4-cyclohexanedione in ethylene glycol (6 g / 40 g) at about 25° C. for about 60 minutes to The carbons A, B and C are treated separately. In addition, the various carbons were treated by contacting a solution of 1,3-cyclohexanedione in ethylene glycol (1 g / 50 g) at about 25° C. for about 120 minutes. In addition, carbon C was treated by contacting a solution of 1,4-cyclohexanedione in 1,2-propanediol (1 g / 50 g) at about 25° C. for about 60 minutes. The results of the surface area analysis are shown in Table 2. As shown, various combinations of diketones and diols provide reduced micropore surface area and macropore surface area, and more specifically, provide preferably reduced micropore surface area.
[0372] Table 2
[0373] sample
[0374] Carbon C
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com