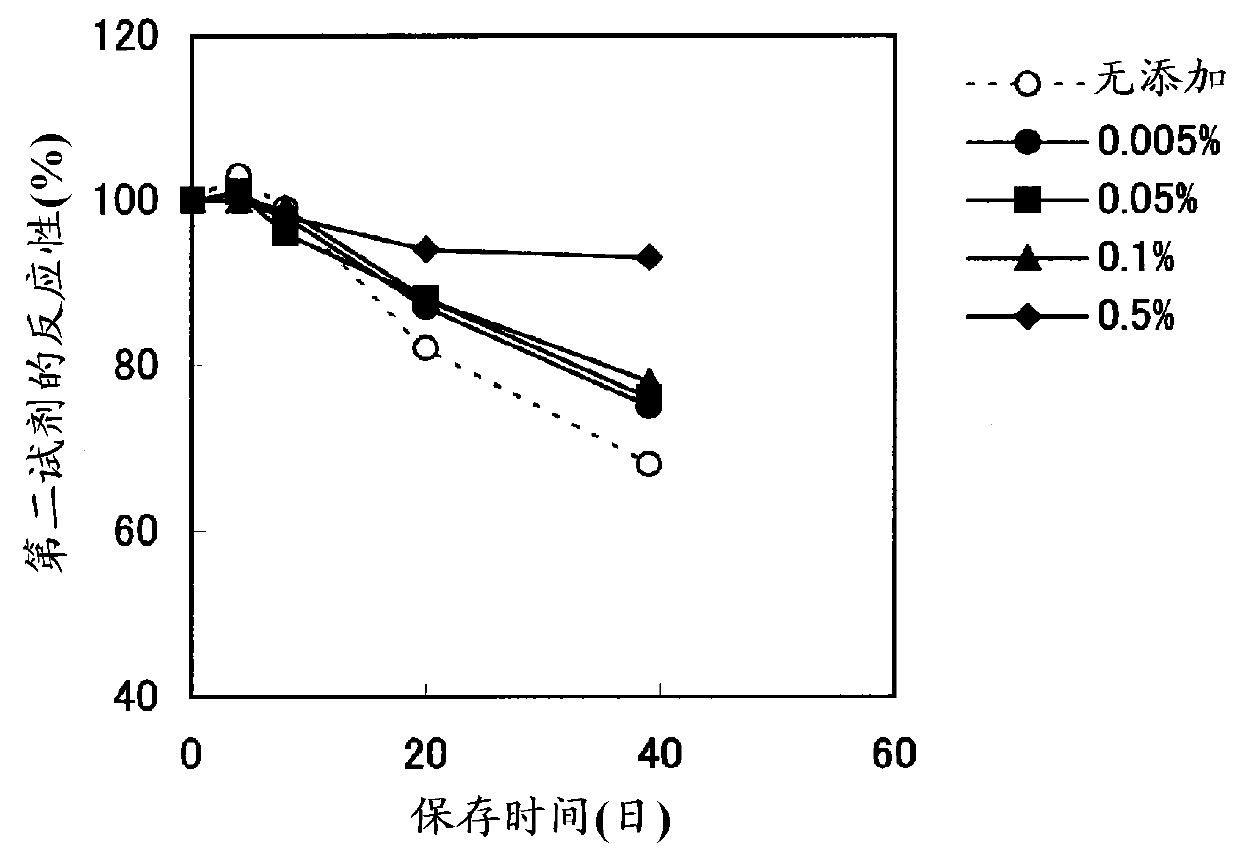
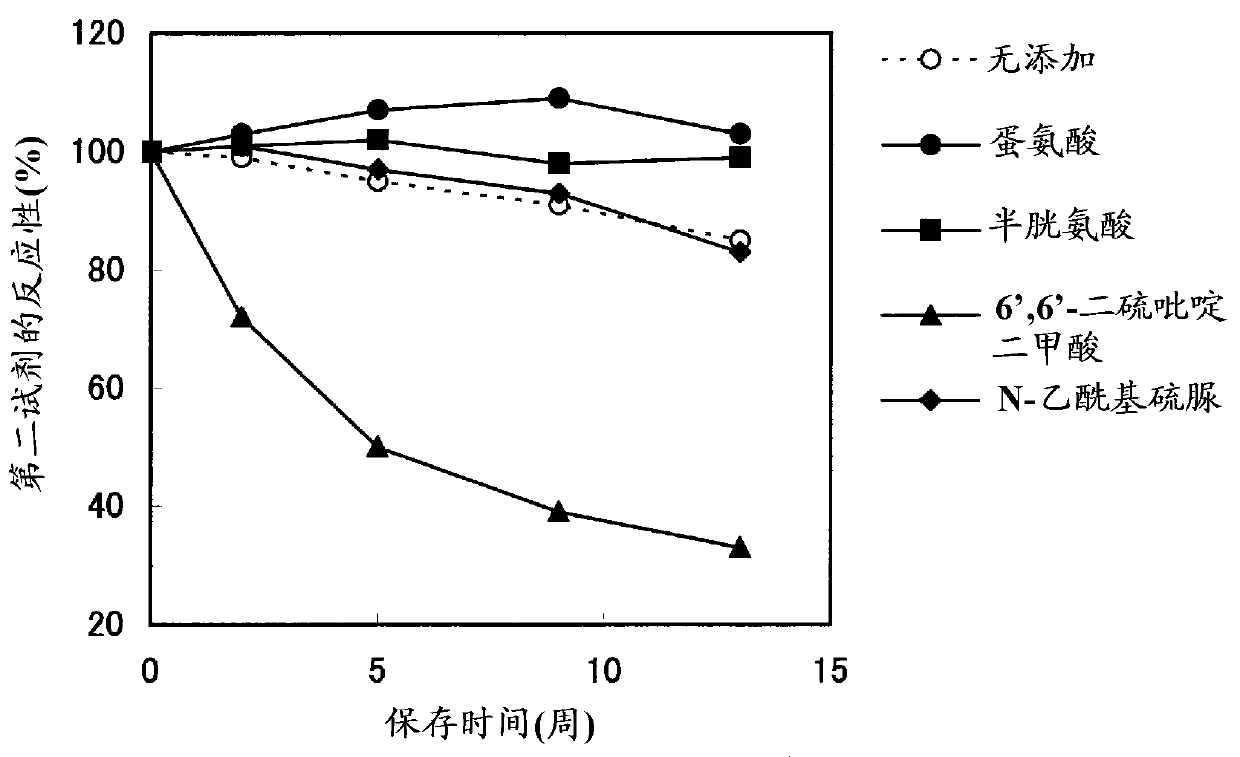
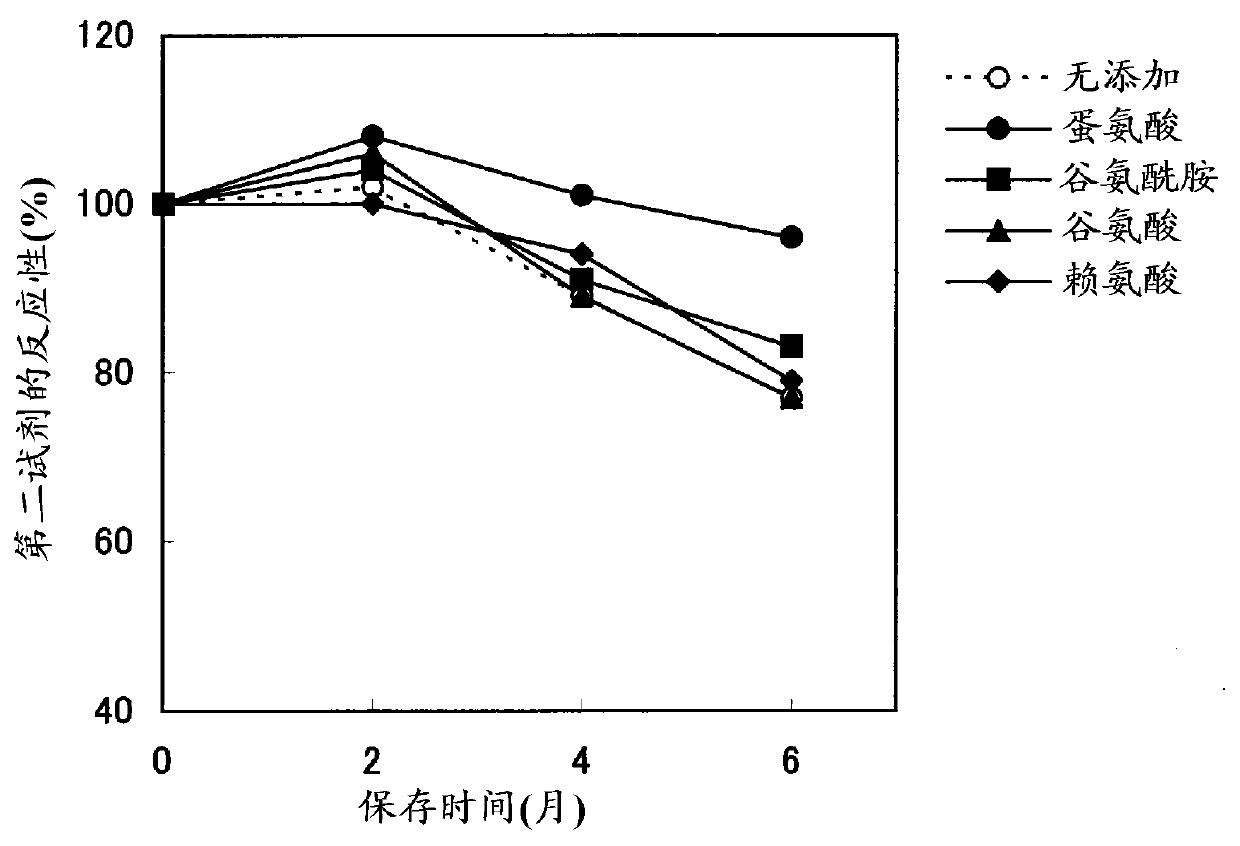
Method for stabilizing microparticles having reactive substance bound thereto, and reagent containing the microparticles
A reactive, material technology, applied in the field of reagents for immunoassays, which can solve the problems of undisclosed gold particle stabilization methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0080] Preparation Example 1: Preparation of Colloidal Gold Solution
[0081] While stirring, 2 mL of 10% chloroauric acid aqueous solution was added to 1 L of 95°C distilled water, and after 1 minute, 10 mL of 2% sodium citrate aqueous solution was added, stirred for a further 20 minutes, and then cooled to 30°C. After cooling, adjust the pH to 7.1 with 0.1% potassium carbonate aqueous solution.
preparation example 2
[0082] Preparation Example 2: Preparation of Anti-Cystatin C Antibody Bonded Colloidal Gold Reagent
[0083] Anti-cystatin C antibody (Dako Japan Inc.) was diluted with 10 mM HEPES (pH 7.1) containing 0.05% sodium azide to prepare 50 mug / mL concentration. 100 mL of this anti-cystatin C antibody solution was added to about 1 L of the colloidal gold solution prepared in Preparation Example 1, and stirred for 2 hours under refrigeration. Further, 110 mL of 10 mM HEPES (pH 7.1) containing 5.46% mannitol, 0.5% bovine serum albumin (BSA), and 0.05% sodium azide was added, and stirred at 37° C. for 90 minutes. Then, centrifuge at 8000rpm for 40 minutes, remove the supernatant, then add about 1L of 5mM HEPES (pH7.5) containing 3% mannitol, 0.1%BSA and 0.05% sodium azide (A solution), Antibody-bonded colloidal gold dispersion. Further centrifuge at 8000rpm for 40 minutes, remove the supernatant, disperse the antibody-bonded colloidal gold with A solution containing 1.5% dextran sul...
preparation example 3
[0084] Preparation Example 3: Preparation of Cystatin C Reduced IgG
[0085] Anti-cystatin C antibody (Dako Japan Inc.) was dialyzed against 10 mM HEPES (pH 7.1) (B solution) containing 0.05% sodium azide, 5 mM EDTA·2Na and 150 mM sodium chloride, Then dilute with solution B to make the concentration reach 10mg / mL. 2-Mercaptoethylamine hydrochloride was added to the antibody solution to make the concentration 6 mg / mL, and after dissolution, it was heated at 37° C. for 90 minutes. The 2-mercaptoethylamine hydrochloride was removed using a gel filtration column to obtain an anti-cystatin C reduced IgG.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com