Barium titanate and barium ferrite composite powder with nucleus shell structure and preparation method thereof
A technology for constructing barium titanate and barium ferrite, which is applied in the fields of core-shell structure barium titanate-barium ferrite composite powder material and preparation thereof, ferroelectric-ferromagnetic multifunctional composite powder material and preparation field thereof , can solve the problems of product purity limitation, complicated operation, expensive organic coupling agent, etc., and achieve the effect of strong magnetoelectric coupling coefficient and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
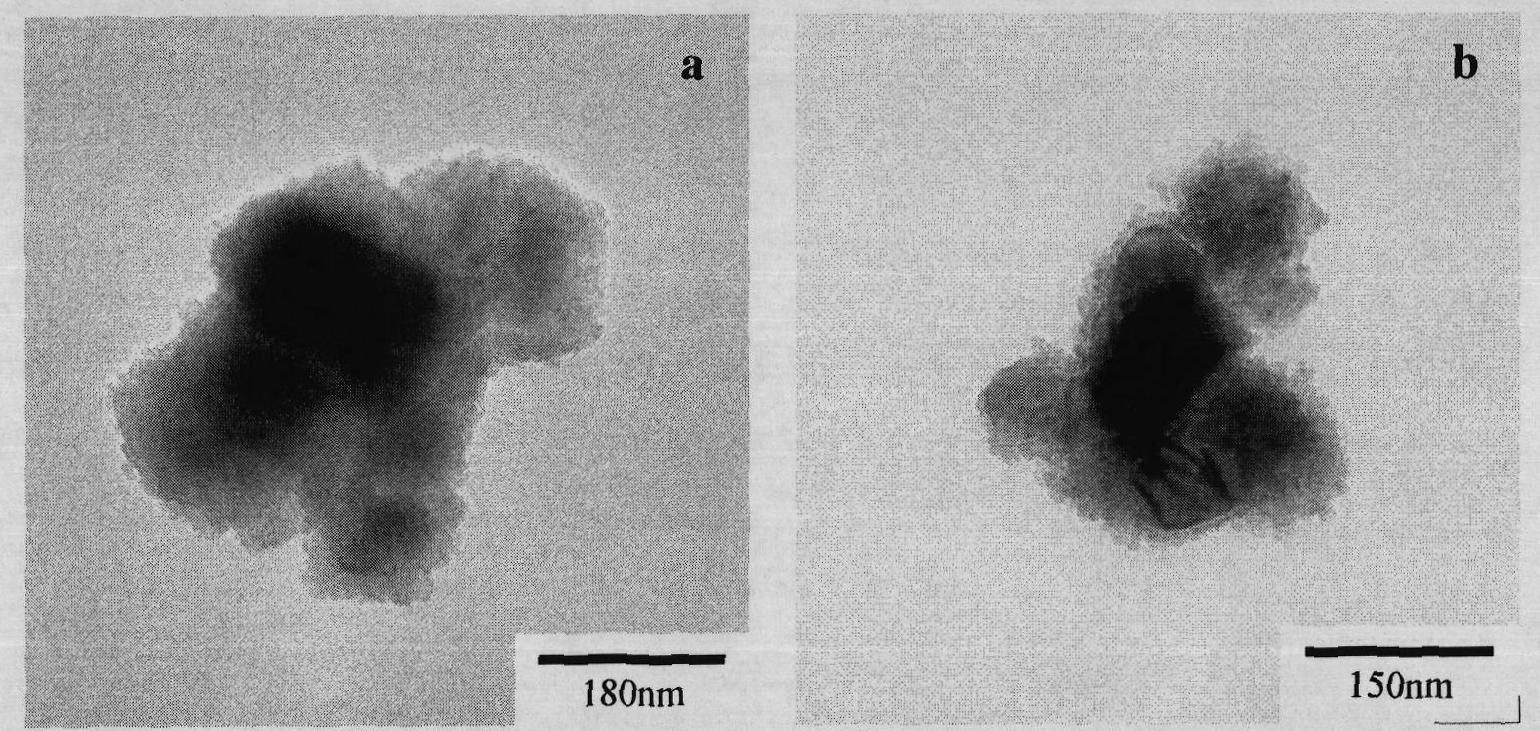
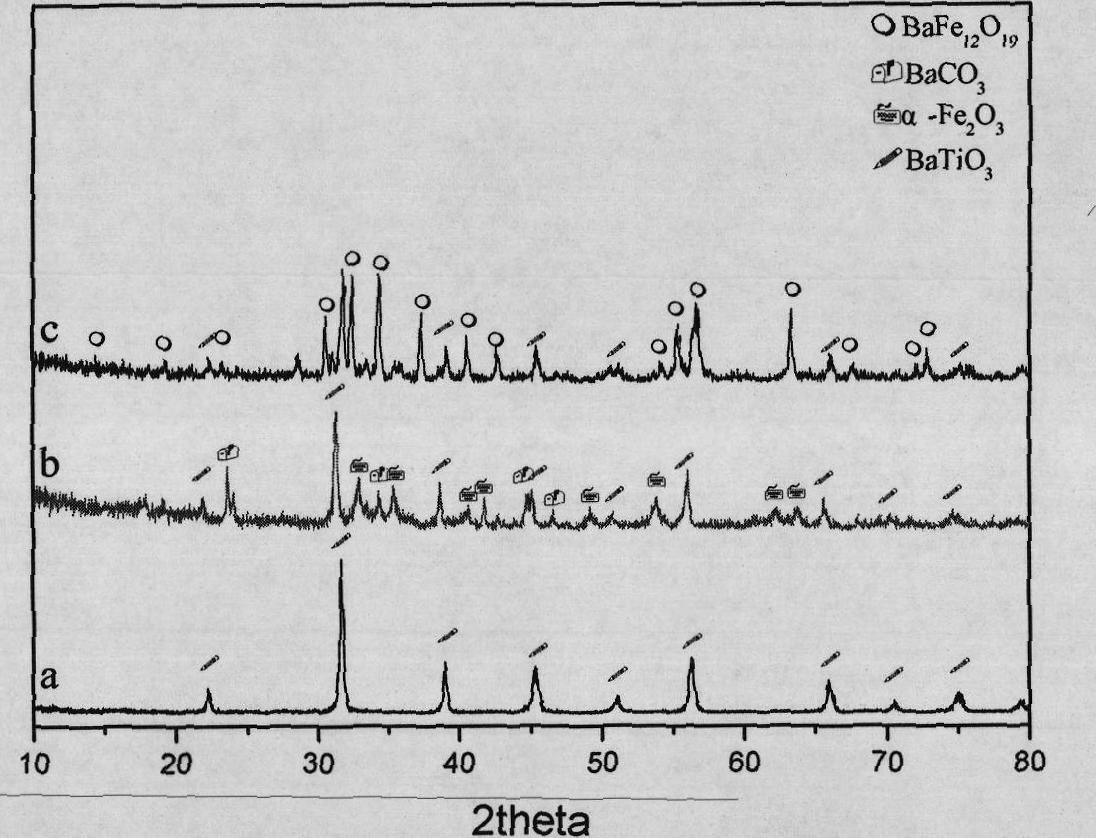
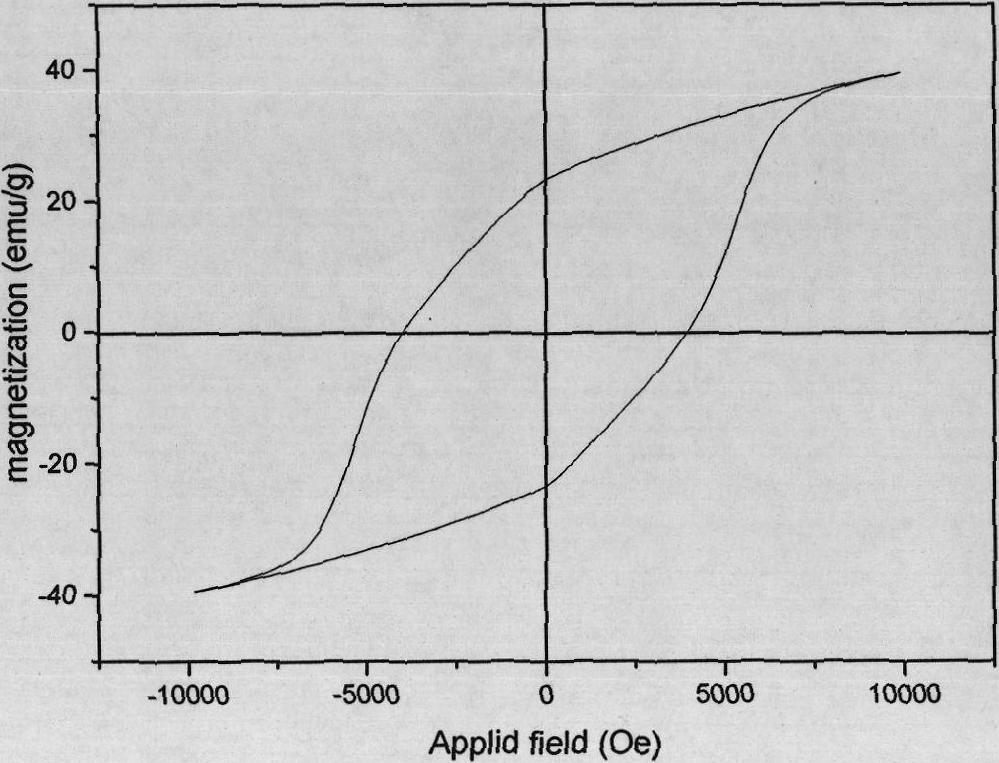
[0034] Weigh an appropriate amount of Fe(NO 3 ) 3 ·9H 2 O, Ba(NO 3 ) 2 And urea are dissolved in deionized water so that the molar concentrations of the three are 0.006mol / L, 0.0005mol / L, 0.8mol / L, respectively, weigh 0.5g of barium titanate powder that has been cleaned and degreasing, and add it to 200ml of the above In the reaction solution, 3wt% polyvinylpyrrolidone was added as a dispersant at the same time. After ultrasonic treatment of the above solution for 30 minutes, it was placed in a closed container and reacted under stirring conditions. The reaction temperature was 100°C and the reaction time was 12 hours. After the reaction, the resulting suspension was centrifuged at 6000 rpm to remove After the supernatant, add ultrapure water to redisperse, and then centrifuge, first use deionized water, then use absolute ethanol to repeat the above process three times, and put the coated precursor after cleaning and separation into a vacuum drying oven at 80℃. Drying for 12h; ...
Embodiment 2
[0036] Weigh an appropriate amount of Fe(NO 3 ) 3 ·9H 2 O, Ba(NO 3 ) 2 And urea are dissolved in deionized water so that the molar concentrations of the three are 0.006mol / L, 0.0005mol / L, 0.8mol / L respectively. Weigh 0.1g of barium titanate powder that has been cleaned and degreasing, and add it to 200ml of the above In the reaction solution, 3wt% polyvinylpyrrolidone was added as a dispersant at the same time. After ultrasonic treatment of the above solution for 30 minutes, it was placed in a closed container and reacted under stirring conditions. The reaction temperature was 100°C and the reaction time was 12 hours. After the reaction, the resulting suspension was centrifuged at 6000 rpm to remove After the supernatant, add ultrapure water to redisperse, and then centrifuge, first use deionized water, then use absolute ethanol to repeat the above process three times, and put the coated precursor after cleaning and separation into a vacuum drying oven at 80℃. Drying for 12h; P...
Embodiment 3
[0038] Weigh an appropriate amount of Fe(NO 3 ) 3 ·9H 2 O, Ba(NO 3 ) 2 And urea are dissolved in deionized water so that the molar concentrations of the three are 0.024mol / L, 0.002mol / L, 0.8mol / L respectively. Weigh 0.5g of barium titanate powder that has been cleaned and degreasing, and add it to 200ml of the above In the reaction solution, 3wt% polyvinylpyrrolidone was added as a dispersant at the same time. After ultrasonic treatment of the above solution for 30 minutes, it was placed in a closed container and reacted under stirring conditions. The reaction temperature was 90° C. and the reaction time was 24 hours. After the reaction, the resulting suspension was centrifuged at 6000 rpm to remove After the supernatant, add ultrapure water to redisperse, and then centrifuge, first use deionized water, then use absolute ethanol to repeat the above process three times, and put the coated precursor after cleaning and separation into a vacuum drying oven at 80℃. Dry for 12h; Put ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com