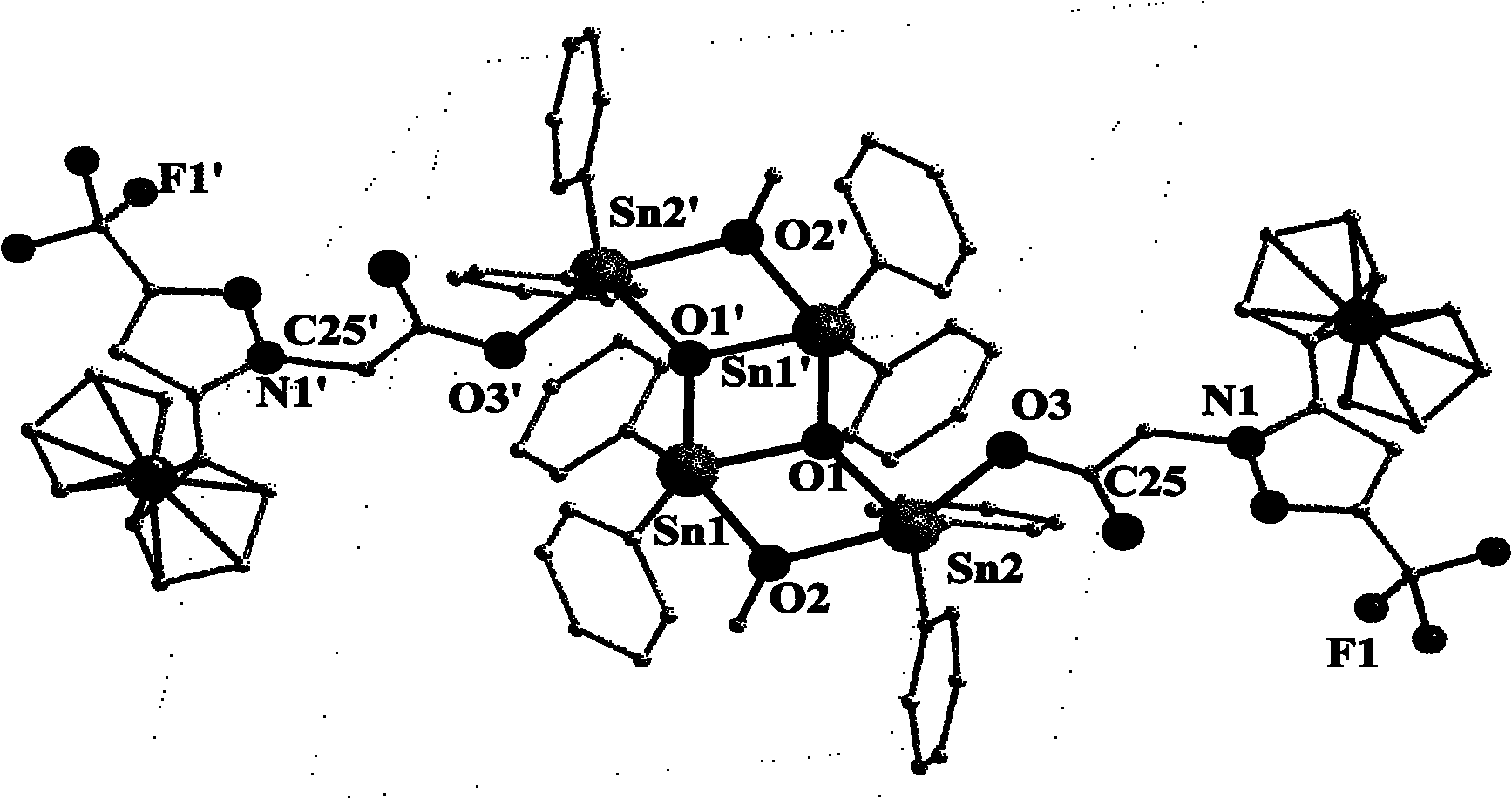
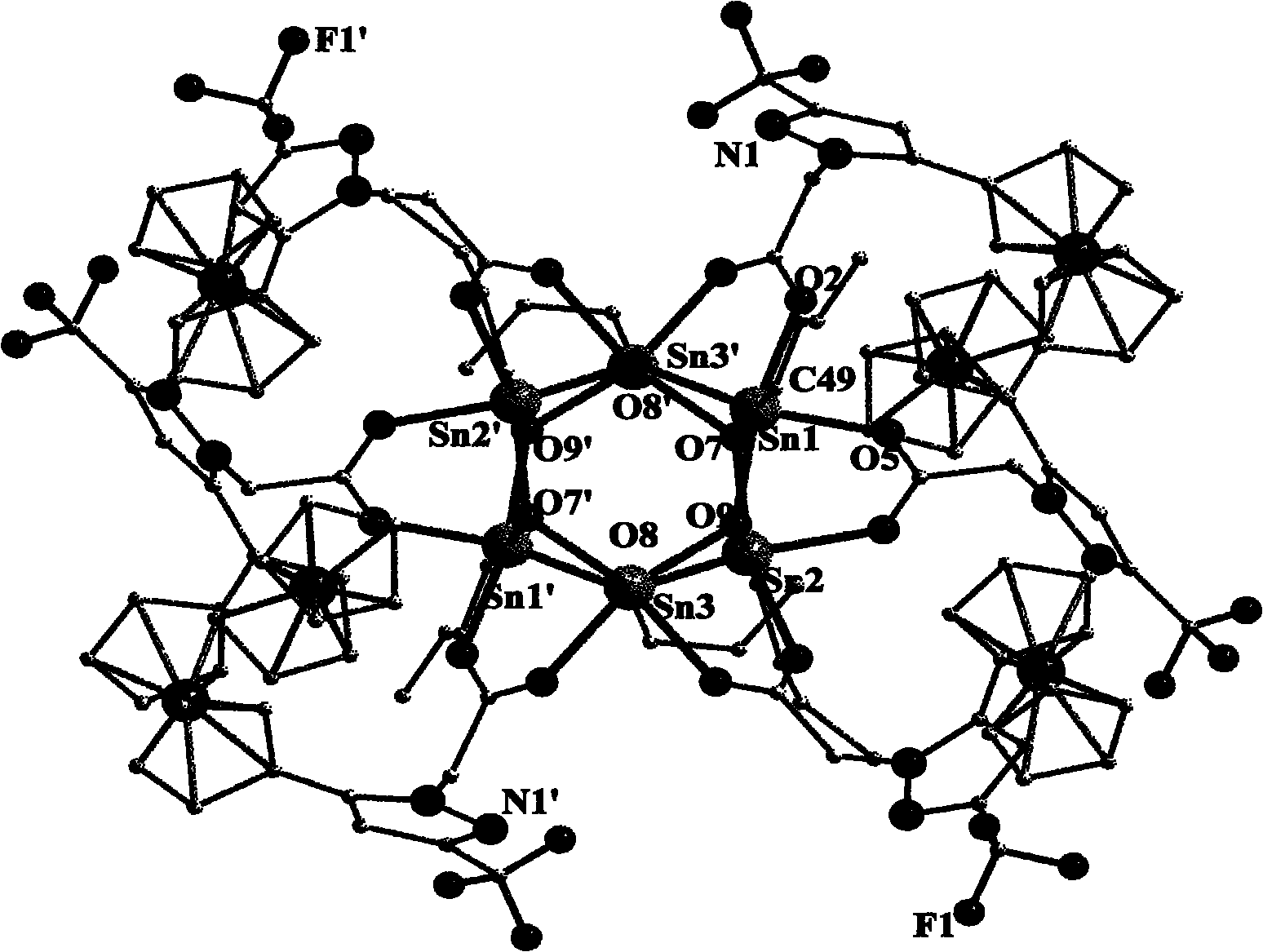
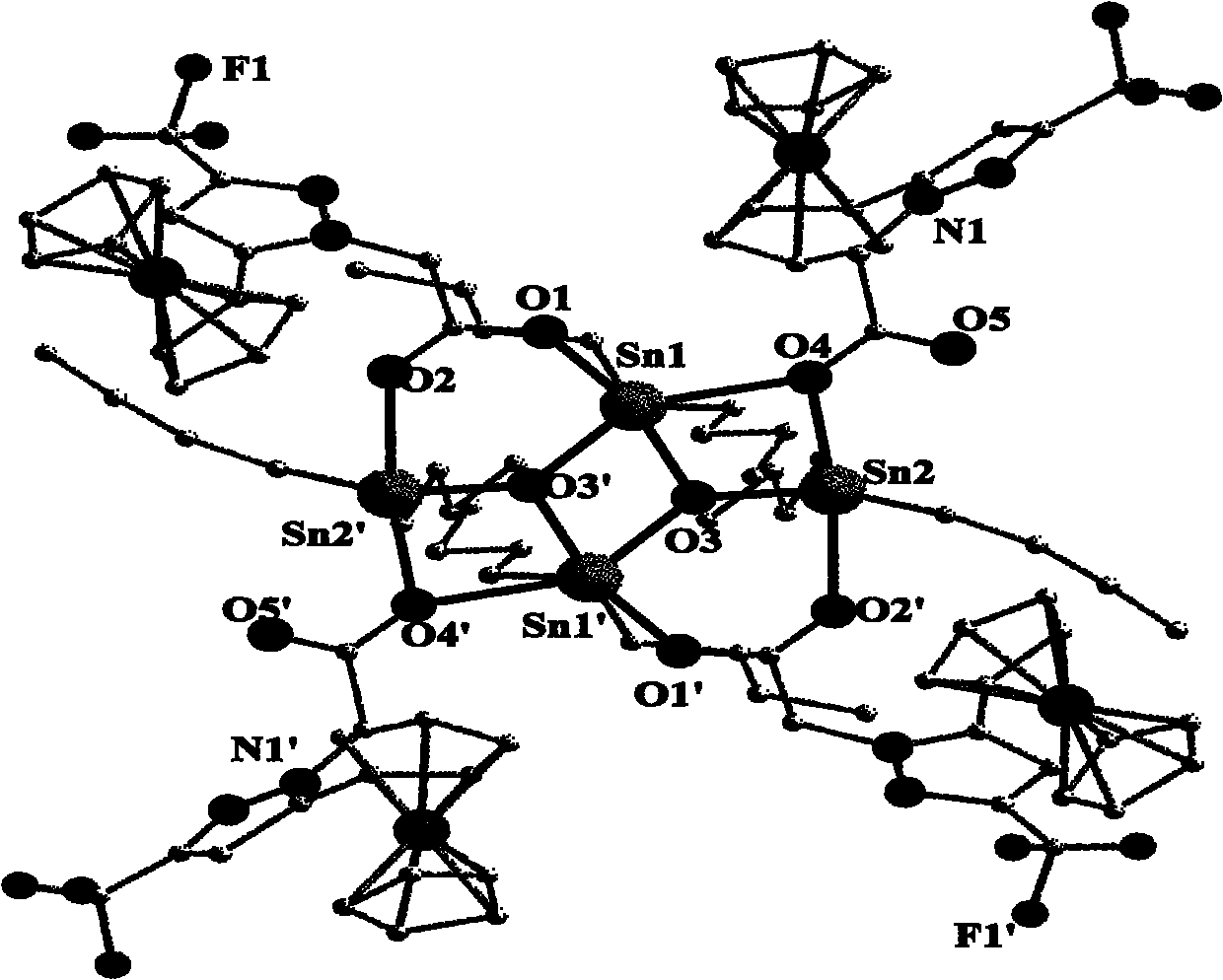
Organotin-oxygen cluster containing ferrocene pyrazole and application of cluster
A technology of ferrocene pyrazolyl and organotin, which is applied in the fields of tin organic compounds, organic chemistry, metallocene, etc., can solve the problems of damaging the immune system of animals, and the low molecular weight organotin compounds are not suitable for anticancer agents, etc. Low, low toxicity, and the effect of improving biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] The present invention is further described in detail by the following examples, but it should be noted that the scope of the present invention is not limited by these examples.
[0038] 1. Synthesis of ligand [3-trifluoromethyl-5-ferrocene pyrazole acetic acid (LCOOH)]:
[0039] Weigh 5-ferrocenyl-3-trifluoromethylpyrazole S (6.40g, 0.02mol) and dissolve it in acetonitrile, then weigh t-BuOK (3.36g, 0.03mol) and add it to the above solution, 60 After heating at ℃ for 2h, the BrCH 2 COOC 2 h 5 (6.68g, 0.04mol) was dropped into the flask drop by drop, and the reaction was carried out for 4-5 hours after the drop, and the reaction was basically completed by TLC detection. Evaporate acetonitrile under reduced pressure, add water to raise the temperature and reflux to hydrolyze the product, stop the reaction after 1.5h, take out the mixture, add dilute HCl (1M) dropwise to the above mixture under ice bath to adjust the pH of the solution to weak acidity (pH=5~6) . Repea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com