Method for preparing isepamicin and salts thereof
A technology of isopamicin and isoserine, which is applied in the preparation of sugar derivatives, chemical instruments and methods, and production of bulk chemicals, can solve the problems of harsh reaction conditions, high cost, poor selectivity, etc., and achieves easy industrialization, Simple operation and highly selective effects
Inactive Publication Date: 2011-06-15
江西制药有限责任公司
View PDF5 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, in this method, the amount of trimethylchlorosilane is very large (at least 8 equivalents), and the cost is high. Moreover, in order to ensure that the silanization is carried out smoothly, the humidity needs to be strictly controlled in the reaction process, and the reaction conditions are harsh. It is not conducive to industrialization, and on the other hand, it further increases production costs
In addition, the selective protection of other hydroxyl groups and amino groups except the 1-position amino group by trimethylchlorosilane is very difficult to achieve in actual industrial production
CN101469007A discloses using gentamicin B as a raw material to react with zinc pivalate, and then using 2-formylmercaptobenzothiazole as an amino protecting agent to selectively protect the 3,6'-position amino group of gentamicin B, Then connect trifluoroacetyl-protected (S)-isoserine on the 1-amino group by amidation reaction, and obtain the product after deprotection. In addition to the aforementioned shortcomings, this route also uses a large amount of ether as a solvent for many times. High toxicity, not suitable for large-scale industrial production
Therefore, the methods described in the prior art have poorer selectivity, are more complicated, and the reaction conditions are too harsh, the reaction is not easy to monitor, the toxicity is high, the yield is low, and / or the cost is high, which is not conducive to the application in industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
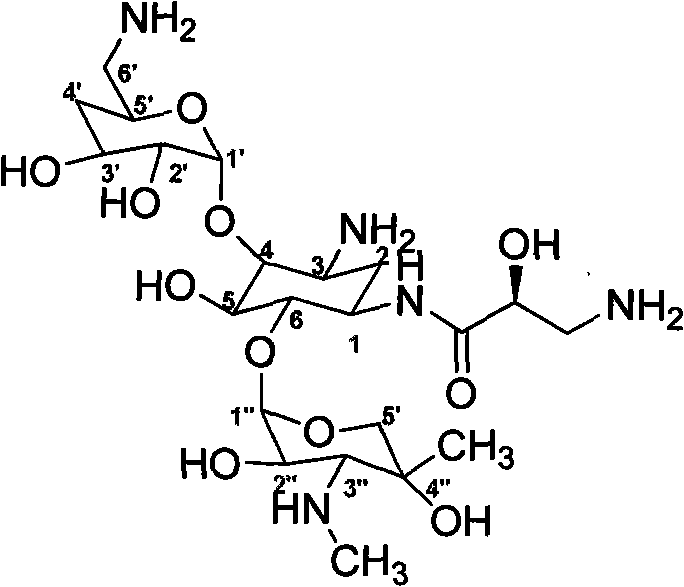
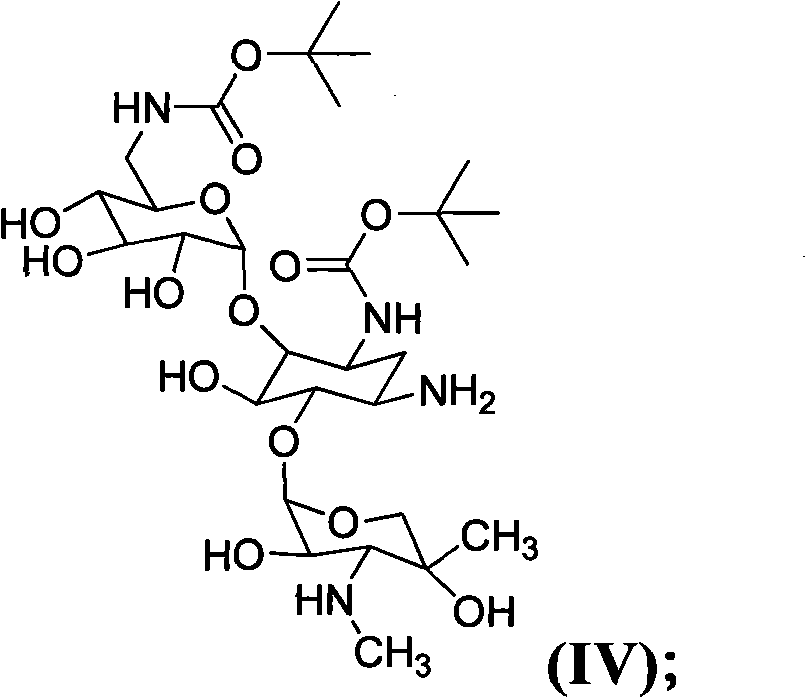
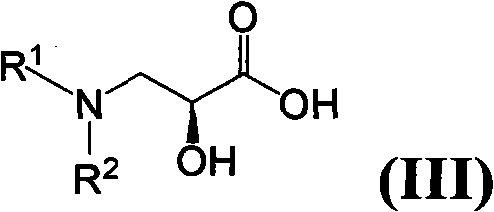
The invention relates to a method for preparing isepamicin for medicinal purpose, which comprises the following steps: (i) performing chelation reaction of gentamicin B and soluble inorganic zinc salt, and performing the amidation reaction of the product of the chelation reaction and butyl dicarbonate to form 3,6'-dibutyloxyacyl gentamicin B; (ii) performing the condensation reaction of the 3,6'-dibutyloxyacyl gentamicin B and nitrogen protected (S)-isoserine to form nitrogen protected isepamicin; and (iii) the removing the nitrogen protective group from the nitrogen protected isepamicin to form isepamicin. The invention also relates to a method for preparing isepamicin salts. The method of the invention has the advantages of high selectivity, high yield, low cost, simple operation, easy industrialization and the like.
Description
A kind of method for preparing isopamicin and salt thereof technical field The invention relates to a method for preparing isopamicin and its salts. Background technique Isepamicin is a known aminoglycoside antibiotic used in the treatment of various diseases caused by bacterial infections, it is stable to various aminoglycoside inactivating enzymes produced by bacteria, therefore, for many pairs of gentamicin Isopamicin is still sensitive to strains that are resistant to antibiotics and amikacin. Isopamicin is still widely used in the treatment of various bacterial infections when other aminoglycoside antibiotics are gradually withdrawn from the first-line drug. Isopamicin has the following structure: Methods for the preparation of isopamicin have been disclosed in the prior art. It can be mentioned that using gentamicin B as a raw material to react with a metal chelating agent, and then using 2-formylmercaptobenzothiazole as an amino protecting agent to selectively ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07H15/236C07H1/00
CPCY02P20/55
Inventor 张威黎俊刘智
Owner 江西制药有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com