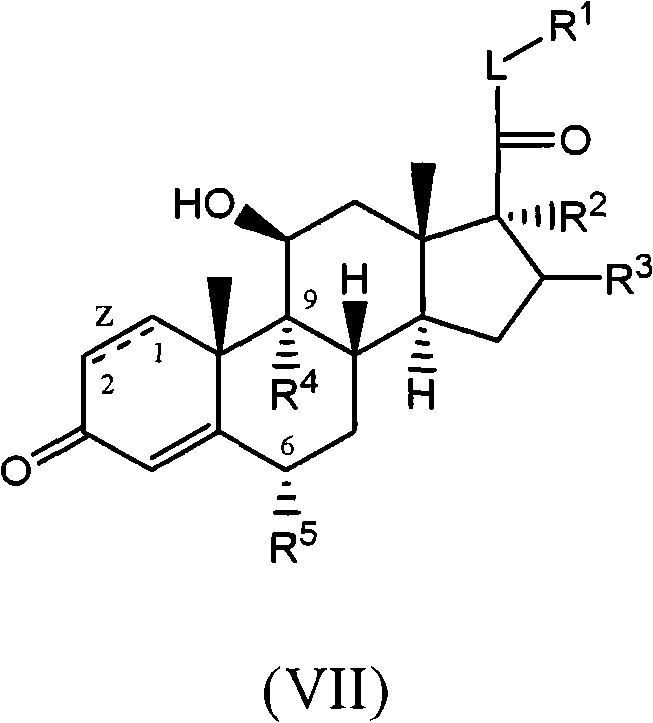
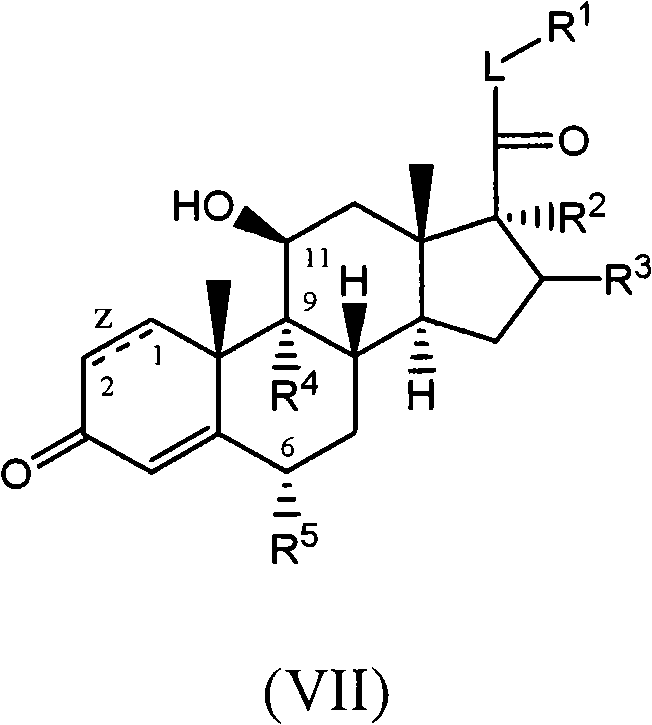
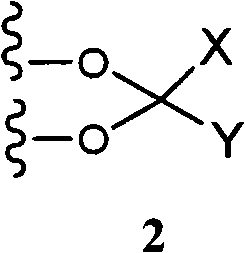
C-21 thioethers as glucocorticoid receptor agonists
A halogen and solvate technology, applied in the field of new C-21 steroid derivatives, can solve the problem of reduced safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0274] In general, the compounds of the present invention can be produced by various methods known to those skilled in the art, for example, by the following methods. These examples should not be considered as limiting the scope of the present disclosure. Alternative mechanistic pathways and analogous structures will be apparent to those skilled in the art.
[0275] Compounds of the invention are most commonly converted by conversion of the C-21 hydroxyl group on the core of a commercially available steroid to a leaving group (e.g. mesylate, triflate) followed by reaction with an appropriate nucleophile (e.g. thiol, alcohol or amine) reaction to prepare (see scheme 1). Commercially available steroid cores can be modified as desired as described in the following examples.
[0276]
[0277] Flowchart 1
[0278] For the purposes of these preparative schemes only, the structures of the compounds are described using simplified terminology. The stereochemistry of the methyl g...
Embodiment 1
[0283] step 1
[0284]
[0285] A solution of hydrocortisone 11 (5 g, 0.0138 mol) in dichloromethane (100 mL) was treated with diisopropylethylamine (8.9 g, 0.0691 mol) at 0°C. The reaction mixture was stirred for 5 minutes; methanesulfonyl chloride (2.9 g, 0.02486 mol) was added dropwise at 0 °C and stirred for 4-6 hours. The reaction mixture was diluted with dichloromethane, transferred to a separatory funnel and washed with dilute HCl, water, brine, and dried over anhydrous sodium sulfate. Removal of solvent gave crude mesylate salt which was purified using column chromatography with dichloromethane and methanol (20:1 ) to afford mesylate salt 12 as a crystalline solid. Yield = 5.5 g (82%).
[0286] step 2
[0287]
[0288] To a solution of hydrocortisone-21-mesylate 12 (10 g, 0.0227 mol) in dichloromethane was added diisopropylethylamine (14.65 g, 0.114 mol) dropwise at 0°C. The reaction mixture was then treated with 2-mercaptobenzothiazole (5.69 g, 0.0341 mo...
Embodiment 2
[0290]
[0291] The title compound was prepared in two steps from desonide 21 using the procedure of Example 1, except that refluxing potassium carbonate in acetone was used in the second step instead of diisopropylethylamine in dichloromethane. The reaction mixture was cooled to room temperature, filtered and the filtrate was concentrated to give crude product. The crude product was purified by column chromatography. M H + 566
[0292] The compounds shown in Table I are non-limiting examples of compounds of the invention synthesized using the procedures described herein (or procedures analogous thereto).
[0293] Table 1
[0294]
[0295]
[0296]
[0297]
[0298]
[0299]
[0300]
[0301]
[0302]
[0303]
[0304]
[0305]
[0306]
[0307]
[0308]
[0309]
[0310]
[0311]
[0312]
[0313]
[0314]
[0315]
[0316]
[0317]
[0318]
[0319]
[0320]
[0321]
[0322]
[0323] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com