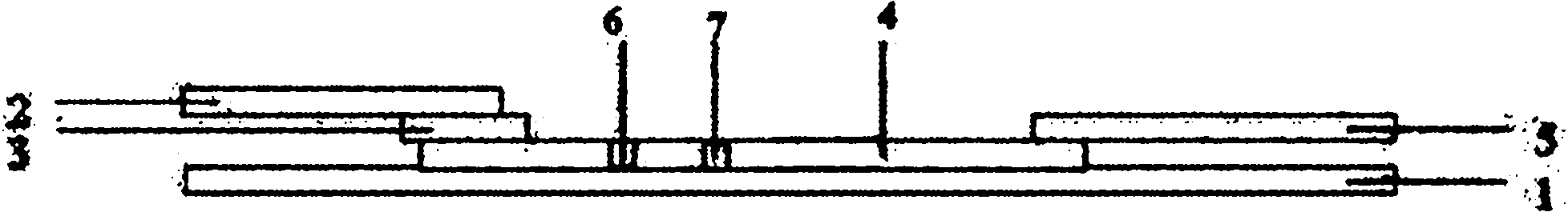Colloidal gold test strip for double-amplification system and preparation method thereof
A technology of colloidal gold test paper and amplification system, which is applied in biological testing, material inspection products, measuring devices, etc., can solve the problem of not further improving the sensitivity of colloidal gold, and achieve the effect of enhancing binding force, improving specificity, and improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
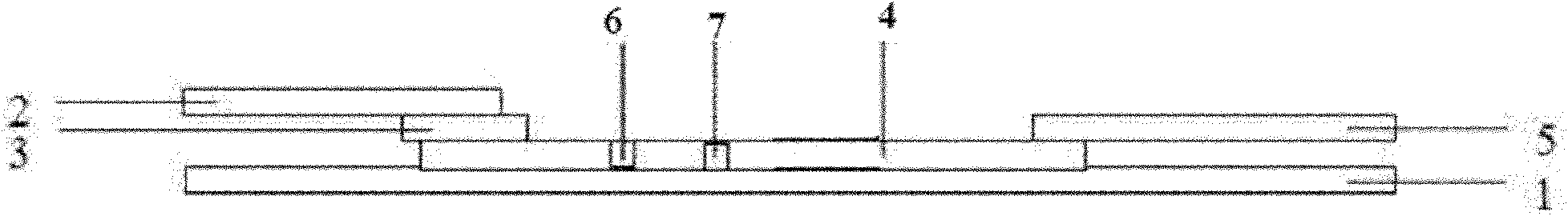
[0023] Such as figure 1 As shown, a colloidal gold test strip of a double amplification system comprises a test strip bottom liner 1, and a sample pad 2 which is overlapped and pasted successively on the test strip bottom liner 1, and a polymer coated with a gold-labeled antibody. Ester film 3, coating film 4 and absorbent paper 5, coating film 4 is coated with detection line 6 and control line 7, and the middle part of coating film 4 is provided with a monoclonal antibody coated with streptavidin-cardiac troponin I in parallel F(ab) of 4C2 2 Fragment test line 6 and a control line 7 coated with rabbit anti-mouse IgG antibody, sample pad 2 coated with F(ab) of mouse cardiac troponin I monoclonal antibody 4C2 2 Fragment-biotin, the gold-labeled antibody is ProteinA labeled with gold particles, through the non-specific binding of ProteinA to the Fc segment of the cardiac troponin I monoclonal antibody M155, the amplified indirect binding of the cardiac troponin I monoclonal ant...
Embodiment 2
[0045] Embodiment 2 adopts the concrete preparation method of the colloidal gold test paper strip of conventional method as follows:
[0046] Cardiac troponin I monoclonal antibody 4C2 and M155 (paired antibody), both purchased from Fitzgerald.
[0047] Preparation of coating film:
[0048] 1) Preparation of coating buffer: filter 0.025M PBS with pH 7.4 through a 0.22u membrane, store at 4°C for use, and have a validity period of 7 days.
[0049] 2) Preparation of blocking solution: PBS containing 1% BSA, 1% sucrose, 0.025M, pH 7.5 was filtered through a 0.22U membrane and placed at 4°C for later use. The validity period was 3 days.
[0050] 3) F(ab) of monoclonal antibody 4C2 2 Fragment preparation: Digest the monoclonal antibody with trypsin, pass through the SPA-SepharoseCL-4B gel chromatography column to remove the Fc fragment, and collect F(ab) 2 fragment.
[0051] 4) F(ab) 2 Preparation of the detection line 6 in section 6: the antibody was prepared at a concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com