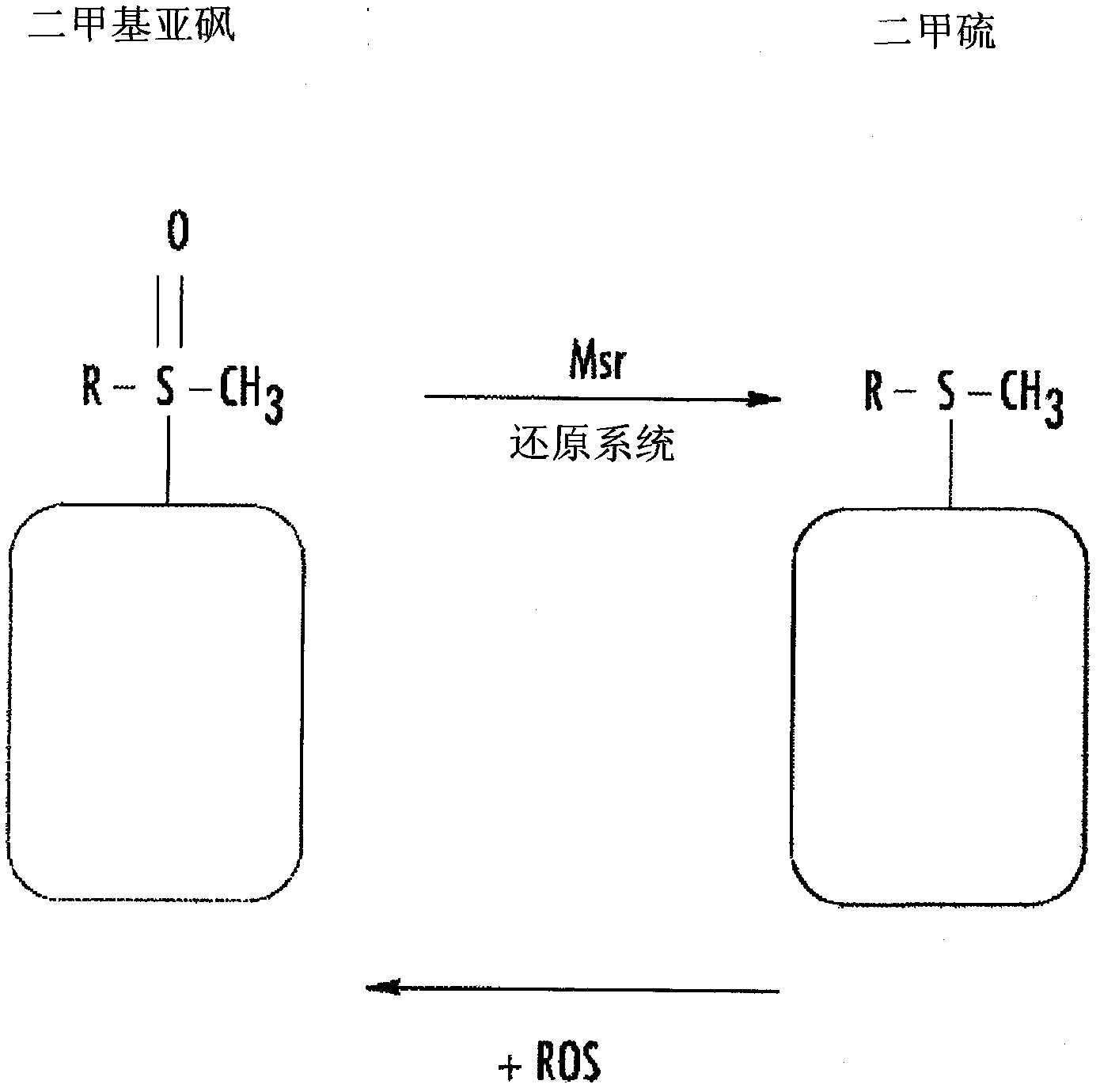
Protection of normal cells
A technology of normal cells and living cells, applied in the fields of pharmacology, medicine, and biochemistry, which can solve problems such as limitation and small ROS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0239] Example 1 - Sulindac as a substrate for MsrA enzyme
[0240] It is known that methionine sulfoxide reductase (MsrA) shows specificity to substrates containing methyl sulfoxide group in S configuration. This example provides evidence that sulindac, an antioxidant known to contain a methylsulfoxide moiety, can serve as a substrate for MsrA.
[0241] Materials and methods
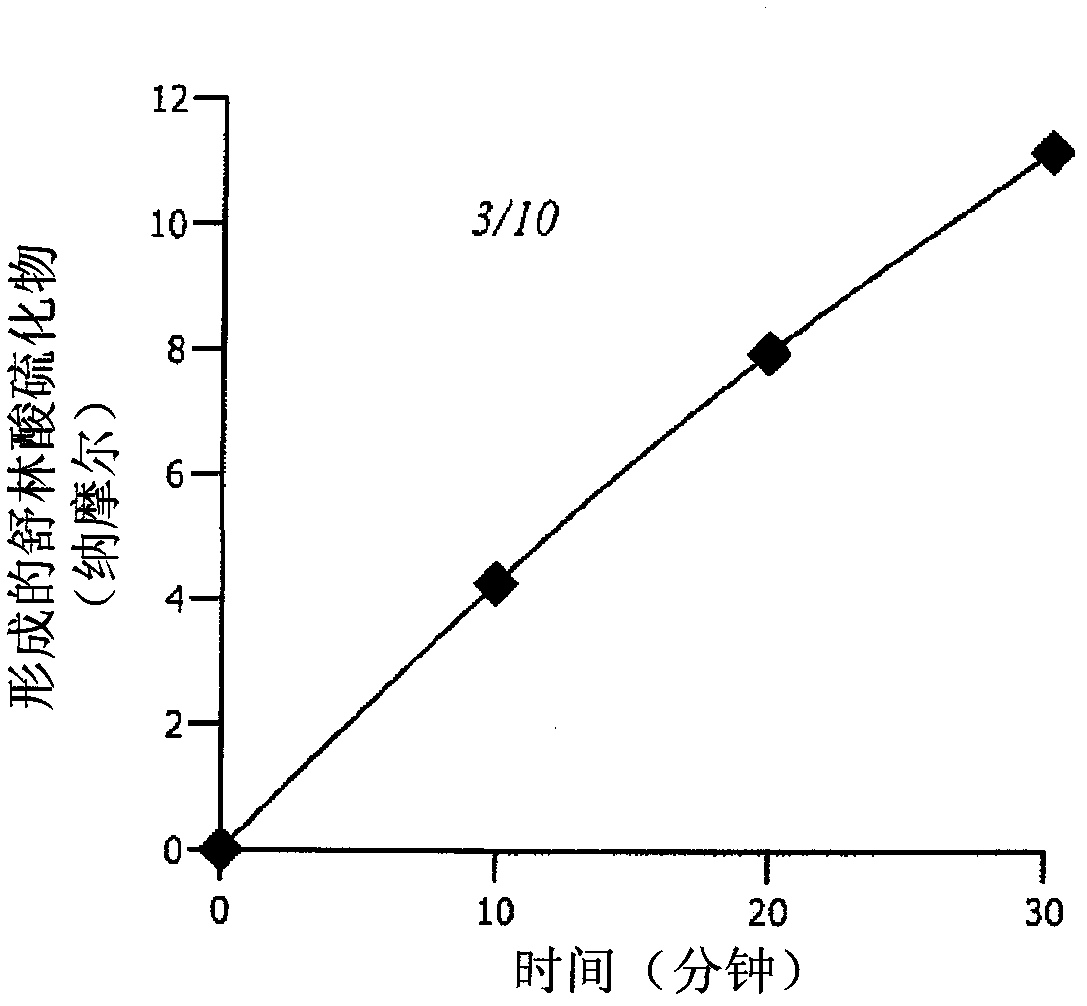
[0242] Reductase test. The reduction of sulindac was measured by a modified NADPH oxidation assay using purified Msr enzyme. Prepare a reaction mixture containing 50 mM Tris-Cl buffer, pH 7.4, 15 µg E. coli thioredoxin reductase, 1 µg E. coli thioredoxin reductase, 100 nmol NADPH, 1 µmole sulindac and 100-400 ng MsrA in a final volume of for 500 μl. Incubate at 37°C for various times.
[0243] The amount of the synthesized product (sulindac sulfide) was determined by measuring NADPH oxidation spectrophotometrically at 340 nm. Since sulindac absorbs strongly at this wavelength, the decrease in abso...
Embodiment 2
[0249] Example 2: Sulindac is a substrate for Msr enzymes in bacteria and mammals
[0250] This example demonstrates that sulindac is a substrate of MsrA and membrane-bound Msr in E. coli, and may be a substrate of other Msr enzymes in mammalian tissues.
[0251] Materials and methods
[0252] Compounds, Enzymes and Substrates. Sulindac (S), sulindac sulfide (SS) and all other compounds and E. coli thioredoxin reductase were obtained from Sigma Chemicals (St. Louis, MO) unless otherwise noted. Thioredoxin (isolated from E. coli) was purchased from Promega (Madison, WI). N-acetyl- 3 H-met-R, S-(O), met-R-(O), met-S-(O) DABS-met-R-(O) and DABS-met-S-(O) according to existing methods Preparation (Brot N. et al., Anal.Biochem.122(1982) 291-294; Lavine, F.T.J.Biol.Chem.169(1947) 477-491; Minetti G. et al., Ital.J.Biochem.43(1994) 273 -283).
[0253]bacterial enzymes. Recombinant MsrA and MsrB from Escherichia coli were obtained using existing methods (Grimaud, R. et al., J. ...
Embodiment 3
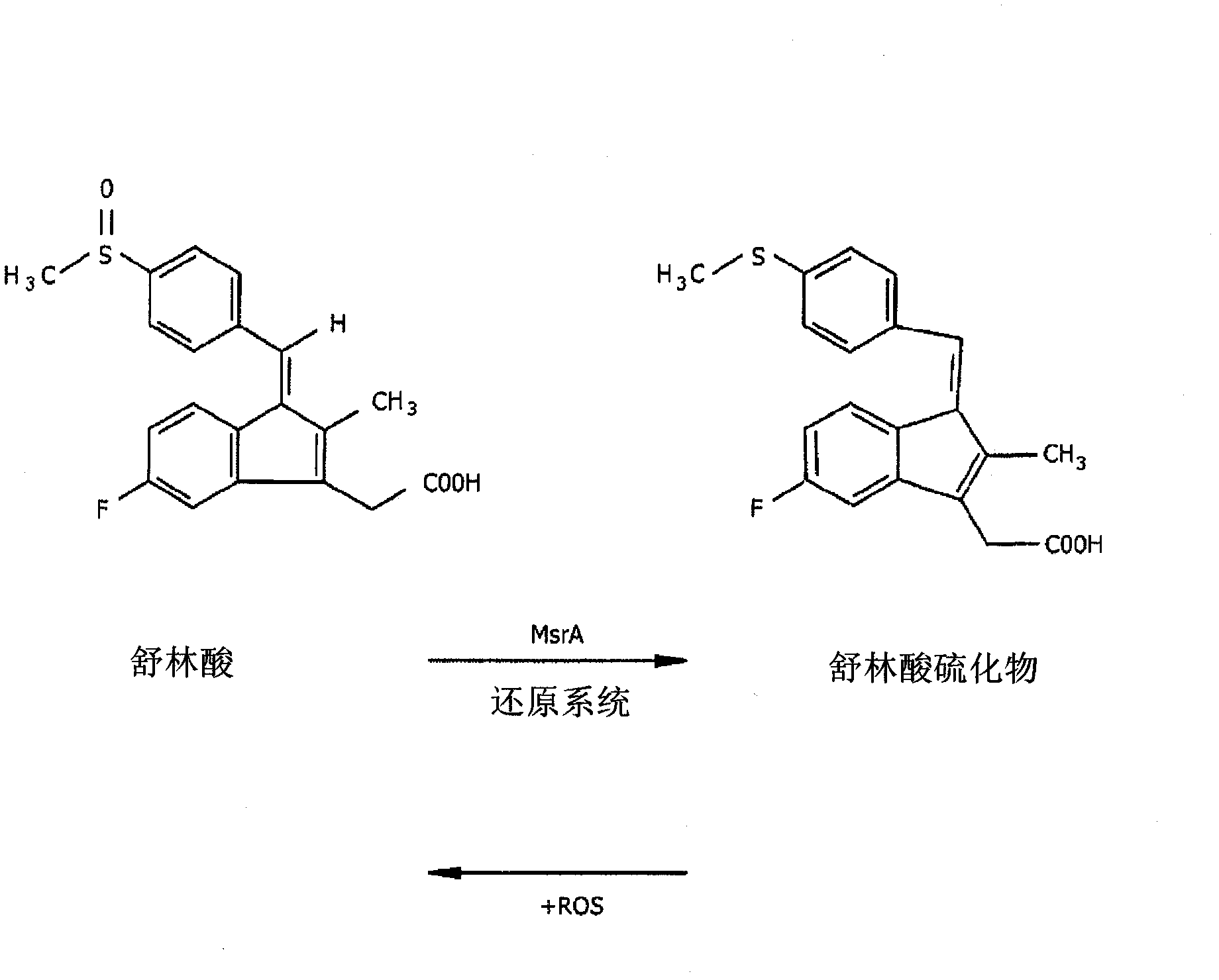
[0282] Example 3 Synthesis of sulindac-methionine-catalyzed antioxidant
[0283] As mentioned above, sulindac is a substrate of MsrA but not MsrB. Sulindac contains a methylsulfoxide moiety that is recognized by the MsrA enzyme, but does not contain an N-methionine sulfoxide moiety (see figure 2 ). This example illustrates the chemical synthesis route of sulindac derivatives. These sulindac derivatives are modified to contain N-substituted methionine, wherein the methionine amino group is a peptide or amide bond. Such sulindac derivatives have Favorable as a substrate for multiple Msr enzymes.
[0284] see Figure 4A, showing that compound 2a (1(Z)-5-fluoro-2-methyl-1-[4-(methylsulfinyl)phenyl]methylene]-1hydro-indene-3-[1- methylthiomethylenyl-2-aminoacetyl]propanoic acid). Compound 2a contains a methionine group linked via an amino group to the acetyl moiety of sulindac. The compound was synthesized as follows starting from sulindac and methionine sulfoxide methyl est...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com