Triterpenoid-based compound used as a virus inhibitor
A compound and composition technology, applied in antiviral agents, plant raw materials, active ingredients of aldehydes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1: the preparation of Japanese alder extract
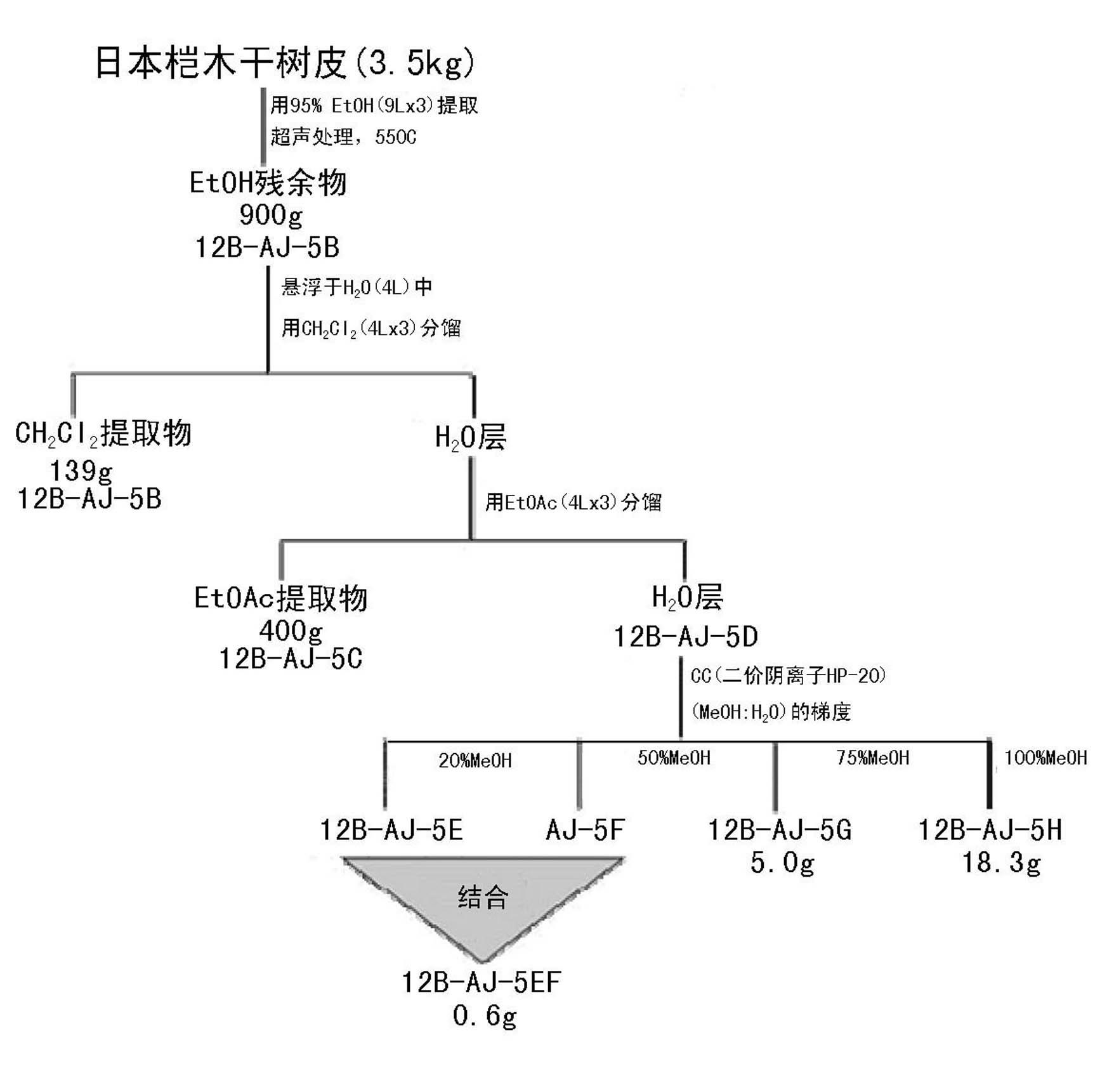
[0100] 3.5 kg of Japanese alder (RNL BIO Co., Ltd.) bark was added to 9 L of 95% ethanol, sonicated three times at 55 °C, and then concentrated to obtain 900 g of ethanol fraction (12B-AJ-5A). Such as figure 1 As shown, the obtained 12B-AJ-5A component is CH 2 Cl 2 and ethanol to obtain dichloromethane (CH 2 Cl 2 ) component (12B-AJ-5B, 139 g), ethanol component (12B-AJ-5C, 400 g) and water component (12B-AJ-5D).
[0101] Furthermore, 12B-AJ-5D was treated with 20%, 50%, 70% and 100% methanol to obtain 12B-AJ-5E, 12B-AJ-5F, 12B-AJ-5G and 12B-AJ-5H .
Embodiment 2
[0102] Example 2: Measurement of antiviral activity of Japanese alder extract
[0103] To measure the antiviral activity of the Japanese alder extract and compounds derived from the Japanese alder extract, KBNP-0028 (KCTC 10866BP), which has excellent proliferation ability, was used as an avian influenza virus. Herein, KBNP-0028 (KCTC 10866BP) was obtained by subculturing A / chicken / Korea / SNU0028 / 2000 (H9N2) isolated in Korea in 2000 and cloning the cultured virus.
[0104] For incubation of hatchery eggshell fragments, the shells of 10–11 day old SPF hatchery eggs (Sunrise Co., NY) were washed with 70% ethanol, and embryos and body fluids were removed. The resulting eggshells were cut to approximately 8 mm x 8 mm size so that the chorioallantoic membrane attached to the inner surface of the eggshell was not detached. Add the cut eggshell fragments to each well of a 24-well culture plate. The medium used in this experiment was prepared by mixing 199 medium (GIBCO-BRL, NY, U...
Embodiment 3
[0110] Example 3: Measurement of Cytotoxicity of Japanese Alder Extracts
[0111] In order to detect whether the Japanese alder extract has cytotoxicity, each organic solvent component prepared in Example 1 (12B-AJ-5A, 12B-AJ-5B, 12B-AJ-5C, 12B-AJ-5D, 12B-AJ-5E, 12B-AJ-5F, 12B-AJ-5G and 12B-AJ-5H, each having a concentration of 12.5, 25, 50 and 100 μg / mL) were added to the MTT solution (0.5% MTT in water) . Then, each solution was added to each well of a 96-well plate in which chicken embryo fibroblasts (CEF) had been cultured. Then, each well of the plate was incubated at 37° C. for 1-3 hours, and then 120 μL of DMSO was added thereto and stirred for 30 minutes. Then, the absorbance of each well was measured at a wavelength of 562 nm using a microplate reader. As a result, 12B-AJ-5A and 12B-AJ-5B fractions showed relatively high cytotoxicity, 12B-AJ-5C fraction showed moderate cytotoxicity, 12B-AJ-5D, 12B-AJ-5E, 12B-AJ- 5F, 12B-AJ-5G and 12B-AJ-5H showed relatively low ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com