Aromatic ring-containing compound for a resist underlayer and resist underlayer composition
By using resist underlayer compositions containing aromatic ring compounds in the microelectronics industry, the problems of high cost and acid contamination of resist underlayer materials in the prior art are solved, and high etching selectivity and multiple etching resistance are achieved. It provides excellent optical and mechanical properties, is suitable for short-wavelength lithography technology, and improves the precision of semiconductor devices.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
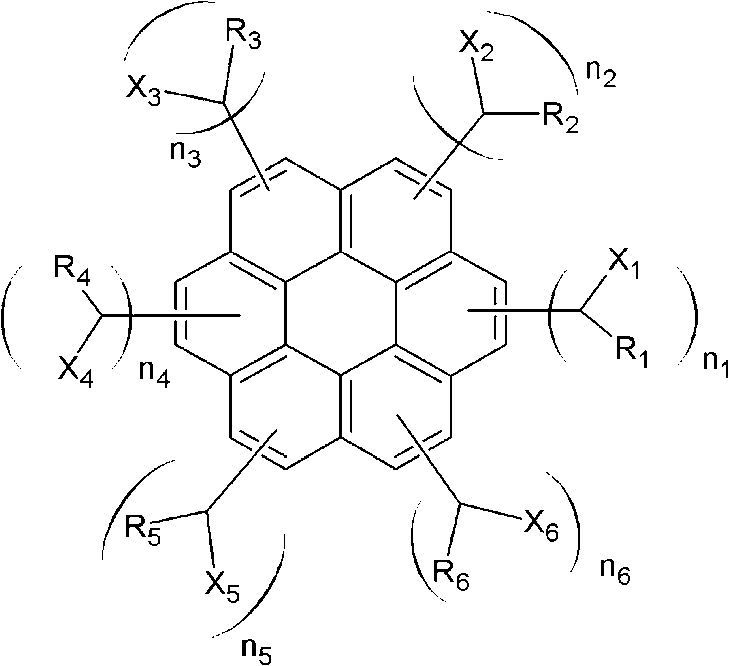
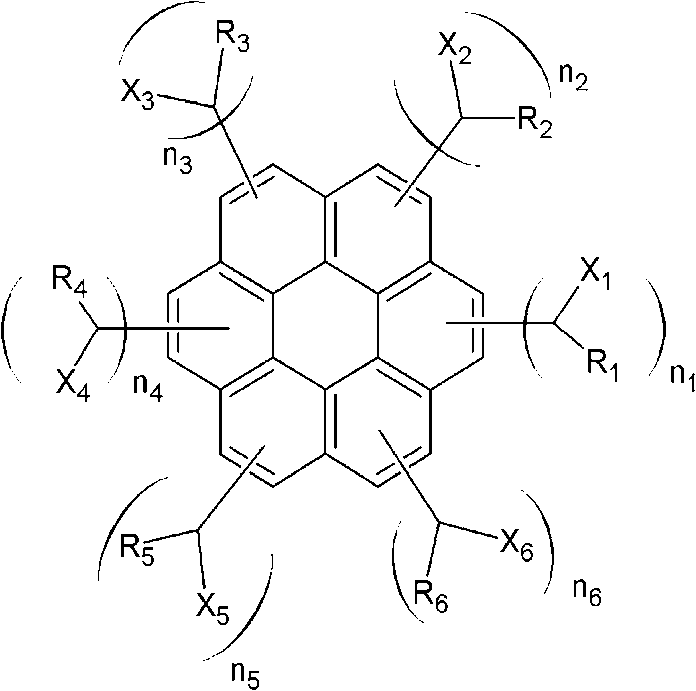
[0057] A solution comprising 30.1 g (0.1 mol) of coronene, 47.1 g (0.6 mol) of acetyl chloride, and 79.8 g (0.6 mol) of aluminum trichloride dissolved in 1000 g of toluene was placed in a place equipped with a mechanical stirrer, Cooler, 2L four-necked flask reactor, and stirring, then the reaction continued for 10 hours. After the reaction was complete, aluminum trichloride was removed using water. To the obtained compound was added 37.83 g (1.0 mol) of sodium borohydride, and then the reaction was continued for 17 hours. After the reaction was completed, reaction by-products were removed using a water / methanol mixture to obtain a compound represented by the following Chemical Formula 2 (average molecular weight=530, 1≤n1+n2+n3+n4+n5+n6≤6).
[0058] [chemical formula 2]
[0059]
Embodiment 2
[0061] A solution containing 30.1 g (0.1 mol) of coronene, 84.32 g (0.6 mol) of benzoyl chloride, and 79.8 g (0.6 mol) of aluminum trichloride dissolved in 1000 g of toluene was placed in a mixer equipped with a mechanical stirrer. , a cooler, a reactor of a 2L four-necked flask, and stirred, and the reaction continued for 10 hours. After the reaction was complete, aluminum trichloride was removed using water. To the obtained compound was added 37.83 g (1.0 mol) of sodium borohydride, and then the reaction was continued for 19 hours. After the reaction was completed, reaction by-products were removed using a water / methanol mixture to obtain a compound represented by the following Chemical Formula 3 (average molecular weight=910, 2≤n1+n2+n3+n4+n5+n6≤6).
[0062] [chemical formula 3]
[0063]
Embodiment 3
[0065] A solution containing 30.1 g (0.1 mol) of coronene, 114.01 g (0.6 mol) of 2-naphtoyl chloride, and 79.8 g (0.6 mol) of aluminum trichloride dissolved in 1000 g of toluene was placed in In a reactor equipped with a mechanical stirrer, a cooler, and a 2L four-necked flask, and stirred, the reaction was continued for 10 hours. After the reaction was complete, aluminum trichloride was removed using water. To the obtained compound was added 37.83 g (1.0 mol) of sodium borohydride, and then the reaction was continued for 19 hours. After the reaction was completed, reaction by-products were removed using a water / methanol mixture to obtain a compound represented by the following Chemical Formula 4 (average molecular weight=980, 2≤n1+n2+n3+n4+n5+n6≤6).
[0066] [chemical formula 4]
[0067]
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| percent by mass | aaaaa | aaaaa |
| percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com