Inorganic fluorescent material excited by blue LED and preparation method thereof
An inorganic fluorescence and blue light technology, applied in the field of fluorescent materials, can solve the problems of poisoned chips and electrodes, unstable performance, complicated preparation, etc., and achieve the effects of no three waste pollution, uniform and fine particle size, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation method of the inorganic fluorescent material provided in this embodiment, the steps are as follows:
[0037] A weighed 2.97mmol, 0.6706g Y 2 o 3 , 12mmol, 2.1186g (NH 4 ) 2 MoO 4 and 3mmol, 0.2216g Li 2 CO 3 , 0.01mmol, 0.0102g Pr 6 o 11 , the doping ratio is 99:1.
[0038] B Mix the precursors, add 50% absolute ethanol liquid of the total mass of the sample, grind and mix.
[0039] C Dry the above product at 80°C in an air blast oven.
[0040] D Place the above precursors in a muffle furnace, increase the temperature programmatically at 500°C, 700°C, and 950°C, sinter for 2 hours, and then grind slightly to obtain the target product.
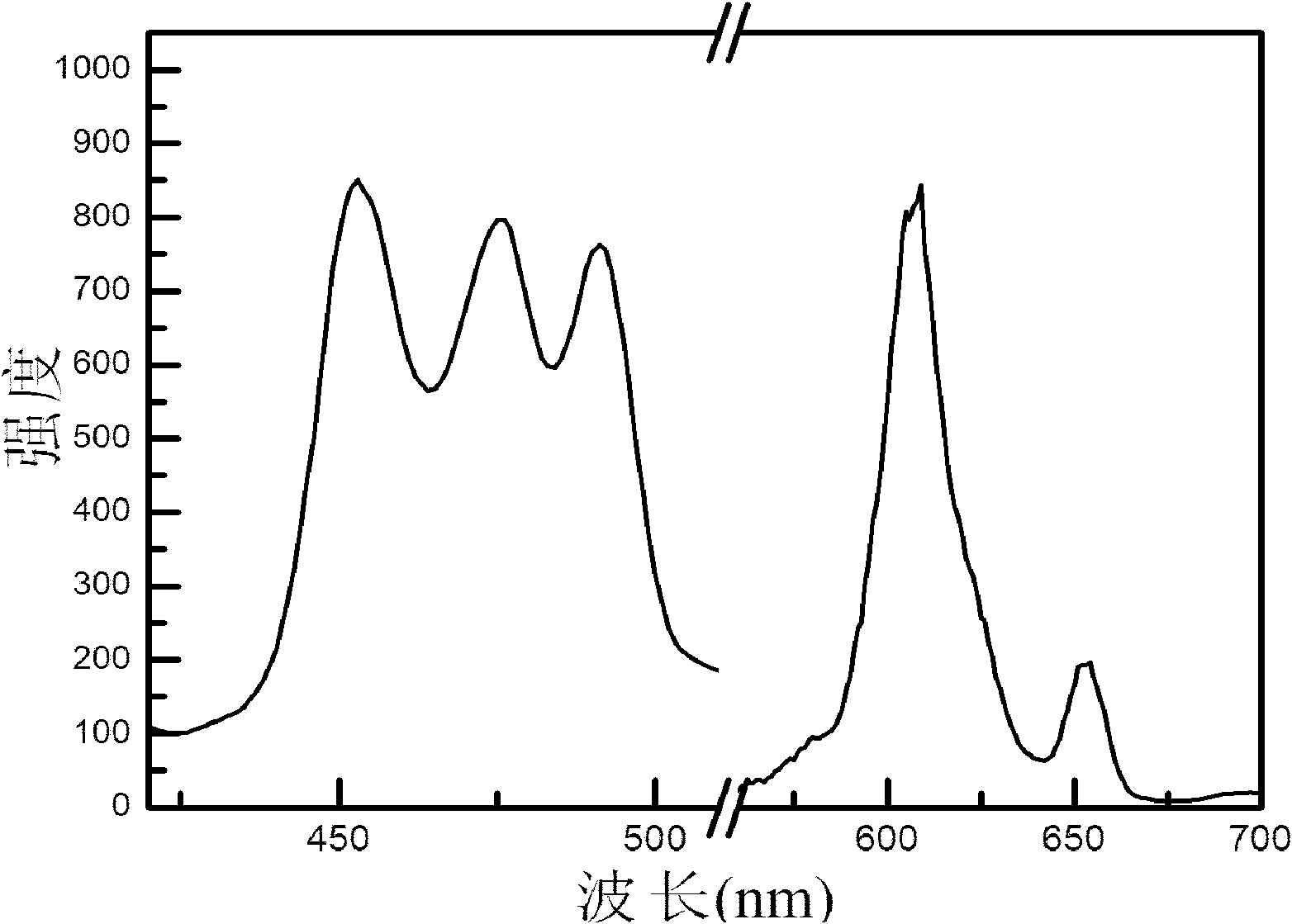
[0041] figure 1 It is the fluorescence spectrum diagram of the inorganic fluorescent material prepared in Example 1; it can be seen from the diagram that the excitation wavelength: 451nm; the emission wavelength: 606nm.
Embodiment 2
[0043] The preparation method of the inorganic fluorescent material of this embodiment, the steps are as follows:
[0044] A weighed 1.485mmol, 0.3353g Y 2 o 3 , 1.485mmol, 0.483.8g La 2 o 3 , 12mmol, 2.1186g (NH 4 ) 2 MoO 4 and 3mmol, 0.2216g Li 2 CO 3 , 0.01mmol, 0.0102g Pr 6 o 11 , the doping ratio is 99:1.
[0045] B Mix the precursors, add 50% absolute ethanol liquid of the total mass of the sample, grind and mix.
[0046] C Dry the above product at 80°C in an air blast oven.
[0047] D Place the above precursors in a muffle furnace, increase the temperature programmatically at 500°C, 700°C, and 950°C, sinter for 2 hours, and then grind slightly to obtain the target product.
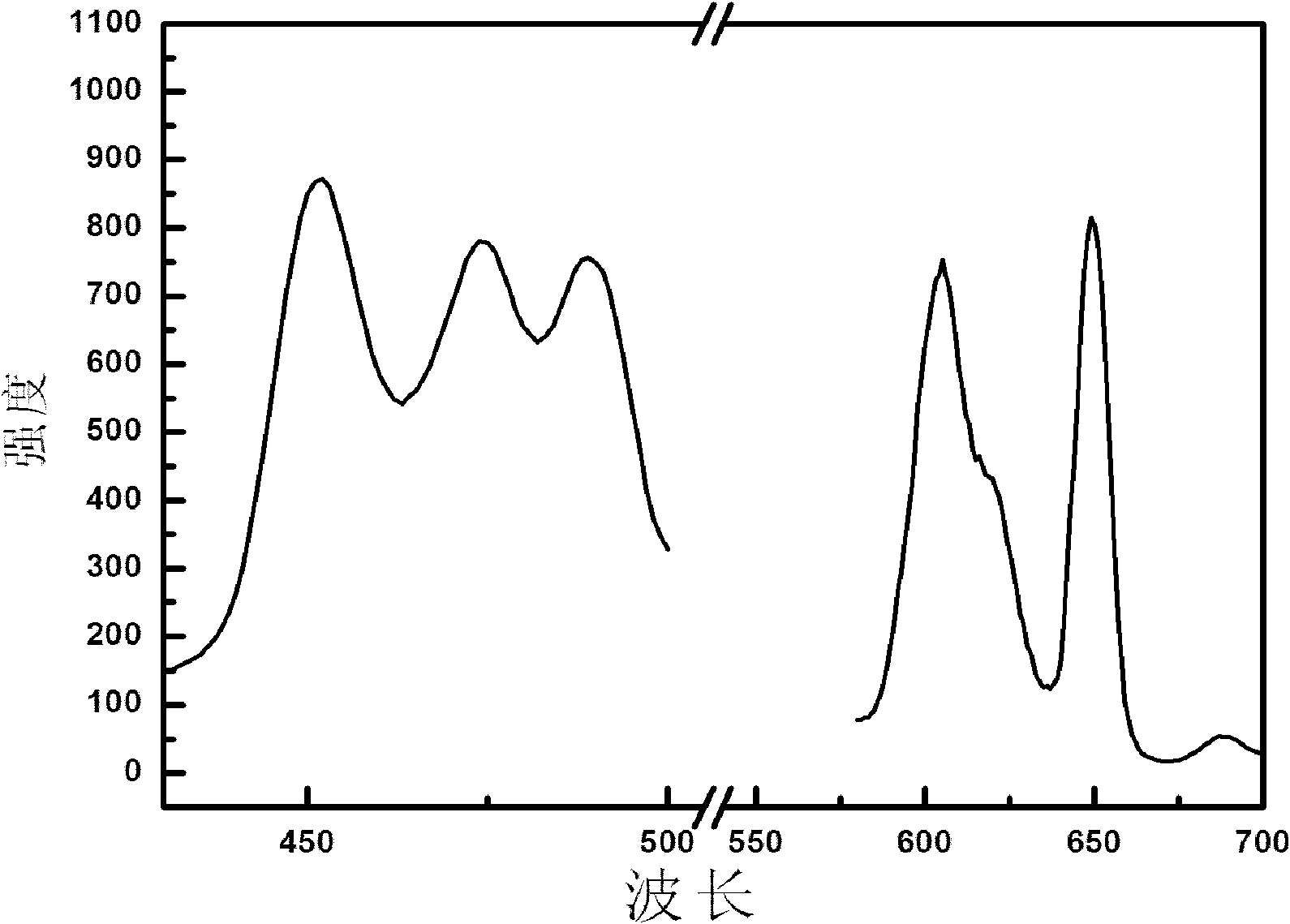
[0048] figure 2 The fluorescence spectrogram of the inorganic fluorescent material prepared for the present embodiment; By figure 2 Visible excitation wavelength: 453nm; emission wavelength: 605nm, 647nm.
Embodiment 3
[0050] The preparation method of the inorganic fluorescent material provided in this embodiment, the steps are as follows:
[0051] A weighed 2.81mmol, 0.6367g Y 2 o 3 , 0.16mmol, 0.0488g La 2 o 3 , 12mmol, 2.1186g (NH 4 ) 2 MoO 4 and 3mmol, 0.2216g Li 2 CO 3 , 0.01mmol, 0.0102g Pr 6 o 11 , the doping ratio is 99:1.
[0052] B Mix the precursors, add 50% absolute ethanol liquid of the total mass of the sample, grind and mix.
[0053] C Dry the above product at 80°C in an air blast oven.
[0054] D Place the above precursors in a muffle furnace, increase the temperature programmatically at 500°C, 700°C, and 950°C, sinter for 2 hours, and then grind slightly to obtain the target product.
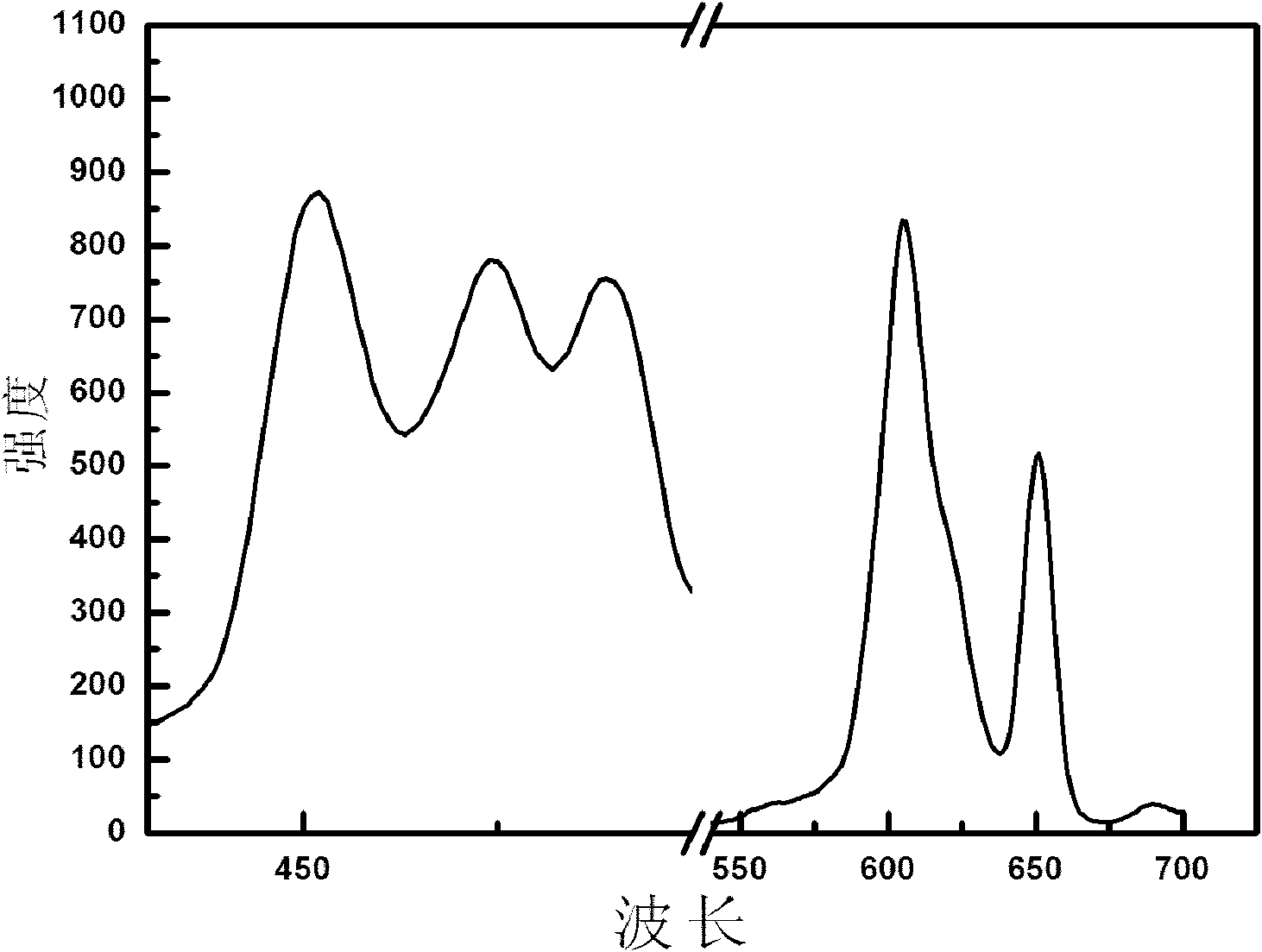
[0055] image 3 The fluorescence spectrogram of the inorganic fluorescent material prepared for the present embodiment; By image 3 Visible excitation wavelength: 451nm; emission wavelength: 605nm, 648nm.
[0056] In addition to the above examples, experiments have proved that: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com