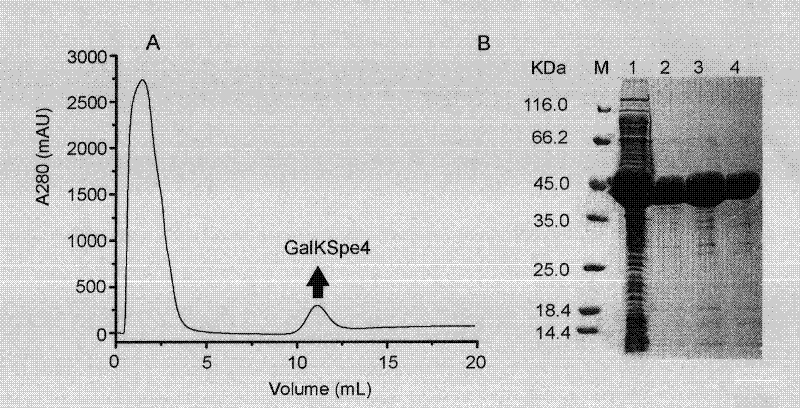
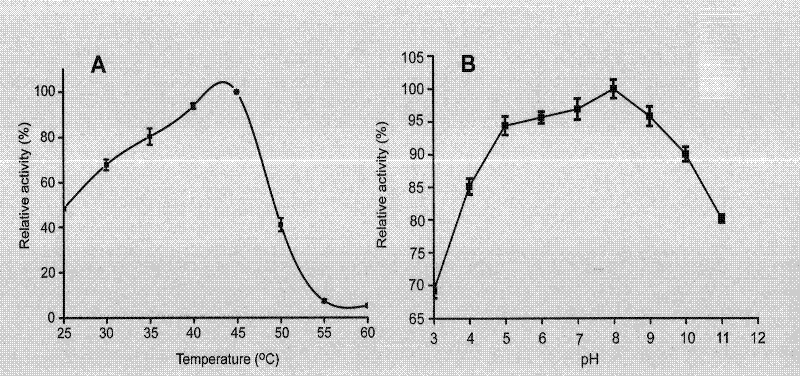
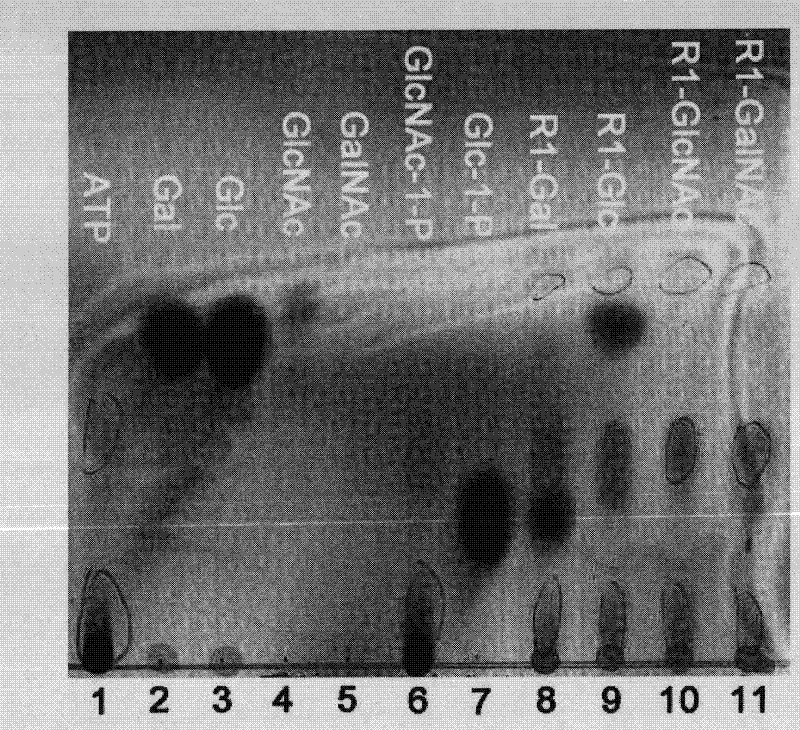
Application of galactokinase in synthesizing N-acetylgalactose-1-phosphoric acid and derivatives thereof
A technology of acetylgalactose and galactokinase, applied in the direction of transferase, fermentation, etc., can solve weak problems and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0049] 1 Materials and methods
[0050] 1.1 Strains and methods
[0051] E.coli DH5α was purchased from Gibco-BRL (Gaithersburg, MD), protein expression strain E.coli BL21(DE3)F-ompT hsdS B (r - B m - B ) gal dcm (DE3) was purchased from Novagen (Carlsbad, CA). The pMCSG7 plasmid was from Novagen (Madison, WI). Reagents and various restriction enzymes used for PCR were from Invitrogen (Carlsbad, CA). Qiagen (Valencia, CA). Plasmid extraction kit and gel recovery kit were purchased from Qiagen (Valencia, CA). Nickel ion affinity chromatography column (5ml) and HiLoad_16 / 60_superdex 200 column were purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
[0052] 1.2 Clone the galK gene from S.pneumonia TIGR4.
[0053] The DNA sequence of the gene galK (GenBank accession No.AAK75925) was extracted from the S.pneumoniae TIGR4 genome, and the PCR primers were as follows:
[0054] GalKS: 5'-TACTTCCAATCCAATGCGATGGCACAACATCTTACT-3' (SEQ ID NO.3)
[0055] GalKA: 5'-TTAT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com