Agent for treating myelofibrosis
A technology of bone marrow and targeting agent, which is applied in bone diseases, biochemical equipment and methods, active ingredients of hydroxyl compounds, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Example 1 : Confirmation of pathological state in mouse model of myelofibrosis
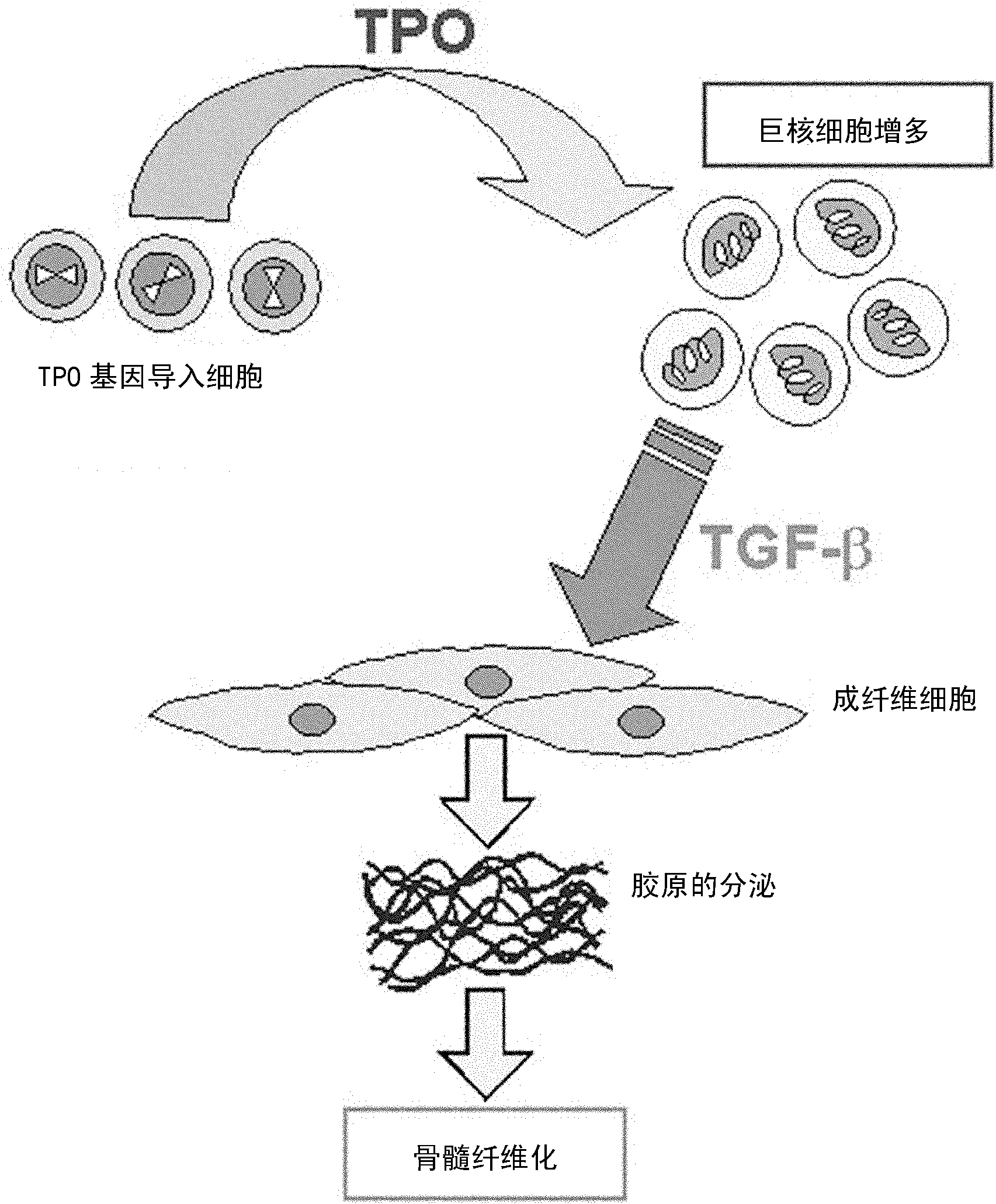
[0102] As a mouse model of myelofibrosis, a transgenic mouse for thrombopoietin (TPO) developed by Dr. Kazuya Shimoda and Dr. Mine Harada (hereinafter also referred to as TPO mouse (see Leukemia Research 29:761-769, 2005)) was used. . In this mouse, due to the excessive production of TPO by TPO gene-introduced cells, the number of megakaryocytes in the bone marrow increases, and the increased megakaryocytes excessively produce transforming growth factor-beta (TGF-beta), which stimulates bone marrow fibrosis. cells, promote the collagen secretion of fibroblasts, and cause myelofibrosis (refer to figure 2 ).
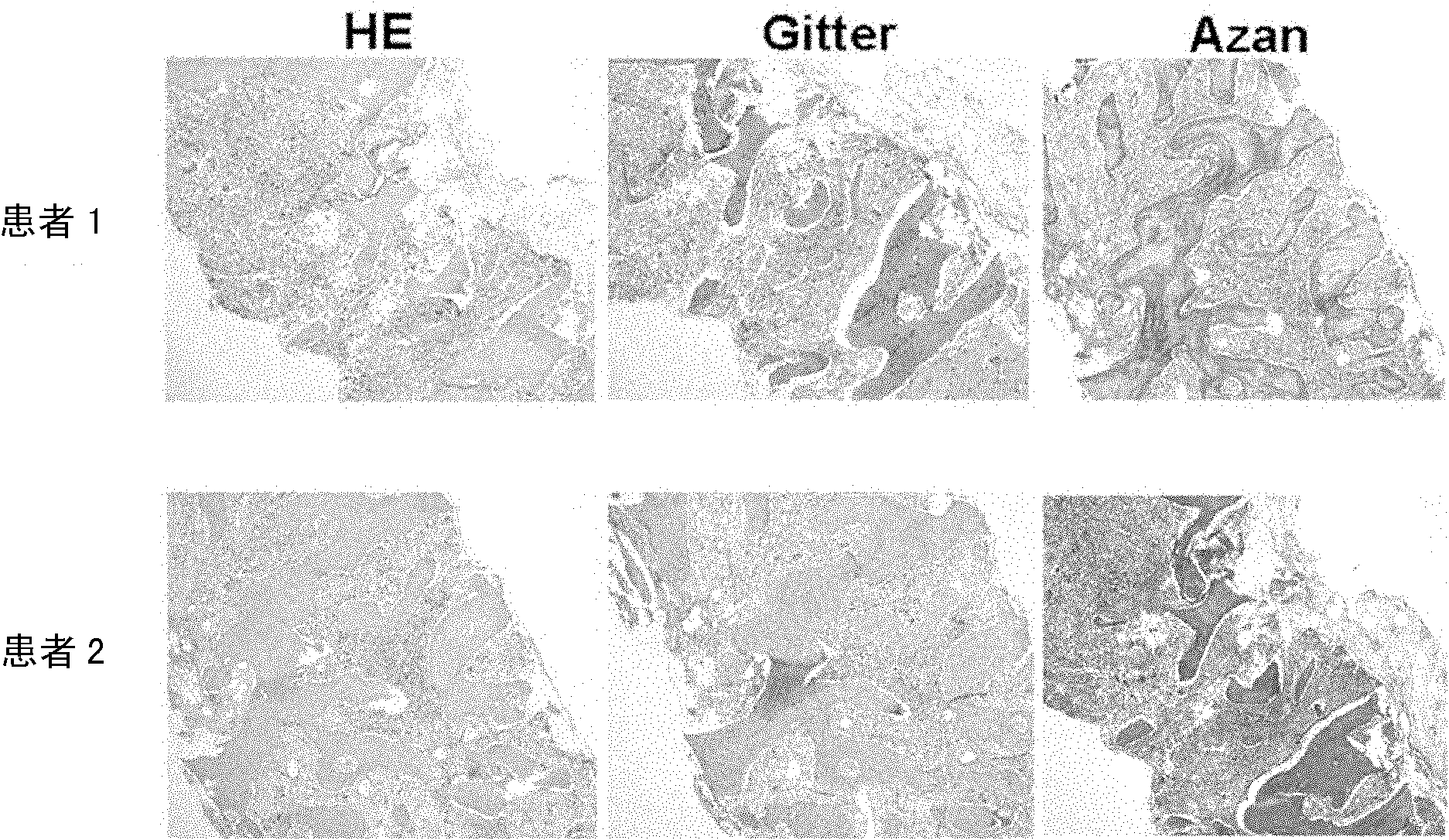
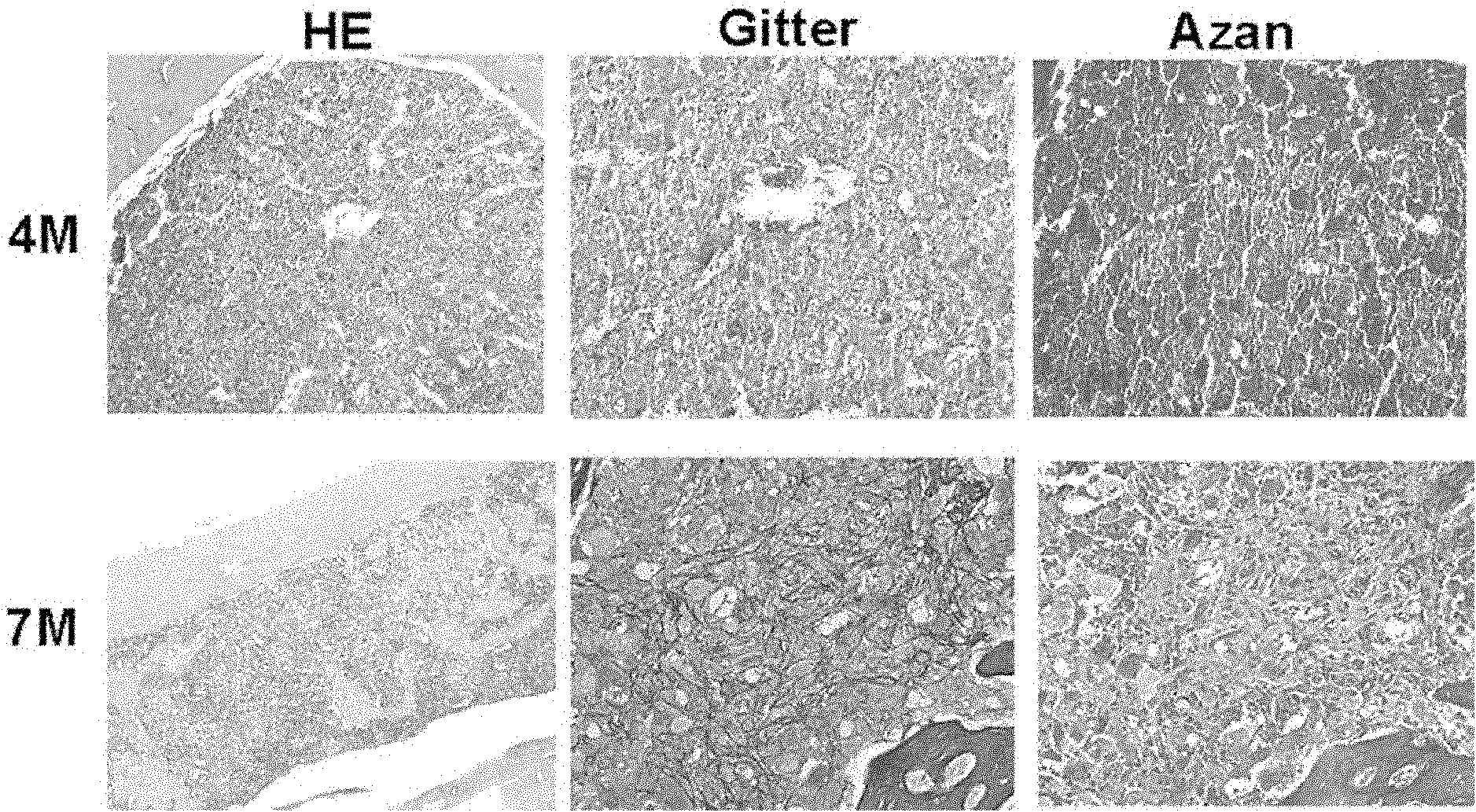
[0103] TPO mice (supplied by the Experimental Facility Department of Kyushu University) were fed with the usual feeding conditions, the mice were euthanized at 4, 7, 9 or 12 months after birth, bone marrow was collected and tissue samples were prepared, and hematoxylin and eosin ( ...
Embodiment 2
[0108] Example 2 : Production of siRNA
[0109]siRNA targeting mouse HSP47 (HSP47 siRNA) was prepared as a drug for inhibiting the activity of extracellular matrix-producing cells in bone marrow. Specifically, five kinds of HSP47 siRNAs (HSP47 siRNA-A to E) and random siRNAs (Random siRNA) having the following sequences were prepared and used in subsequent experiments. Among them, HSP47 siRNA was purchased from iGENE Co., Ltd. (Tokyo) (the target sequence of HSP47 siRNA was designed according to the database Refseq (GenBank Accession No. NM_009825) registered in November 2006). Random siRNA was also purchased from iGENE Corporation (trade name: dsRNA scramble).
[0110] HSP47 siRNA-A: 5'-UGGAUGGGAAAGAUGCAGAAGAAGGAG-3' (sense, sequence number: 1)
[0111] 3'-UAACCUACCCUUUCUACGUCUUCUUCC-5' (antisense, serial number: 2)
[0112] HSP47 siRNA-B: 5'-UGUCUGAGUGGGUAUUUUUAGACAGAG-3' (sense, sequence number: 3)
[0113] 3'-UAACAGACUCACCCAUAAAAAUCUGUC-5' (antisense, sequence number...
Embodiment 3
[0123] Example 3 : Properties of primary cultured bone marrow fibroblasts from TPO mice
[0124] Bone marrow cells from TPO mice aged 4 to 6 weeks were cultured in MEM (Minimum Essential Medium Eagle, Sigma) supplemented with 15% fetal calf serum (FCS) for 4 weeks to obtain primary cultured cells. bone marrow fibroblasts. Figure 6 The cell morphology observed under an inverted microscope is shown, and the cells are typical spindle-shaped fibroblasts. Figure 7 Flow cytometry using the respective antibodies (anti-Vimentin antibody (Santa Cruz Biotechnology), anti-αSMA antibody (Santa Cruz Biotechnology)) was used to analyze the expression of markers vimentin (Vimentin) and αSMA in mesenchymal cells As a result, we can see the expressions of the two. This result shows that the cells cultured above are typical bone marrow fibroblasts. Wherein, the flow cytometer used for analysis is FACS calibur (Becton Dickinson Company), and the measurement data is analyzed using CellQues...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com