Method for preparing 3-chlorine-5-bromophenol
A technology of bromophenol and nitroaniline, which is applied in the field of preparation of 3-chloro-5-bromophenol, can solve the problems such as the preparation method of 3-chloro-5-bromophenol has not been retrieved, and achieves simple and easy to operate post-processing, The effect of high reaction yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
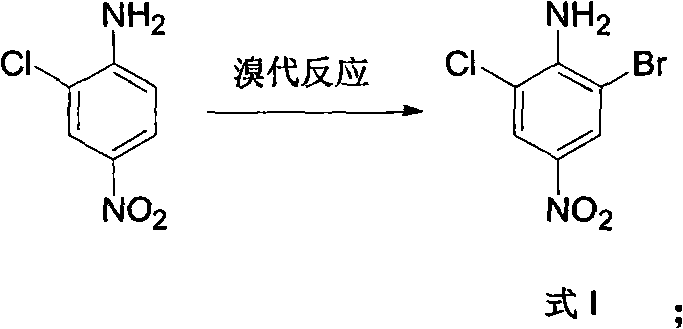
[0035] 1) Bromine substitution reaction:
[0036] Add 69 parts of 2-chloro-4-nitroaniline and 210 parts of glacial acetic acid into the reaction kettle and stir, add 70 parts of liquid bromine dropwise at room temperature, and continue stirring for 2 hours after adding, pour the reaction mixture into another In the reactor of 1000 parts of ice water, a large number of yellow solids were precipitated. The yellow solid was filtered, washed thoroughly with ice water, and dried at room temperature to obtain 90 parts of yellow-green solid product 2-chloro-4-nitro-6-bromoaniline.
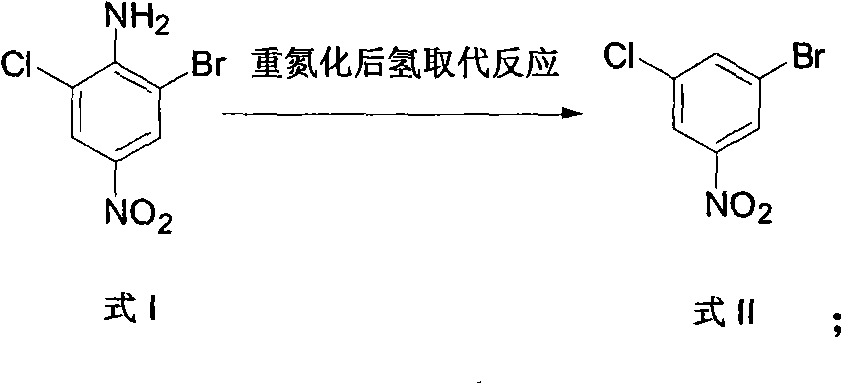
[0037] 2) Hydrogen substitution reaction after diazotization:
[0038] Add the above 90 parts of 2-chloro-4-nitro-6-bromoaniline, 110 parts of concentrated sulfuric acid, and 450 parts of ethanol into the reaction kettle, heat up to 70°C under stirring and react for 30 minutes, and then batch to Add 55 parts of sodium nitrite into the reaction kettle, continue to stir at 70°C for 3 hours after the addit...
Embodiment 2
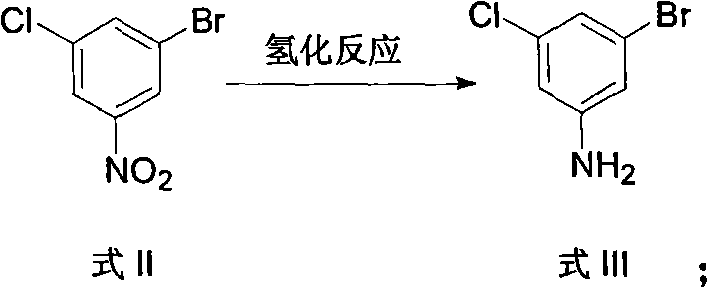
[0045]The steps 1), 2) and 3) were carried out in the same manner as in Example 1 to obtain the diazonium salt solution in 4). Add 70 parts of water and 140 parts of concentrated sulfuric acid into another reaction kettle, then heat up to 80°C, and add the prepared diazonium salt solution dropwise while stirring, stir for 2.5 hours after the dropwise addition, and let it stand to cool to room temperature , extracted twice with methyl tert-butyl ether, then the organic phase was washed with water and then with 20% aqueous sodium hydroxide. Combine the lye and adjust the pH value to 3-4 with concentrated hydrochloric acid. Fully extracted with methyl tert-butyl ether, the organic extract layer was washed with water, dried over anhydrous sodium sulfate, filtered to remove the desiccant, and then precipitated to obtain 25 parts of solid product 3-chloro-5-bromophenol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com