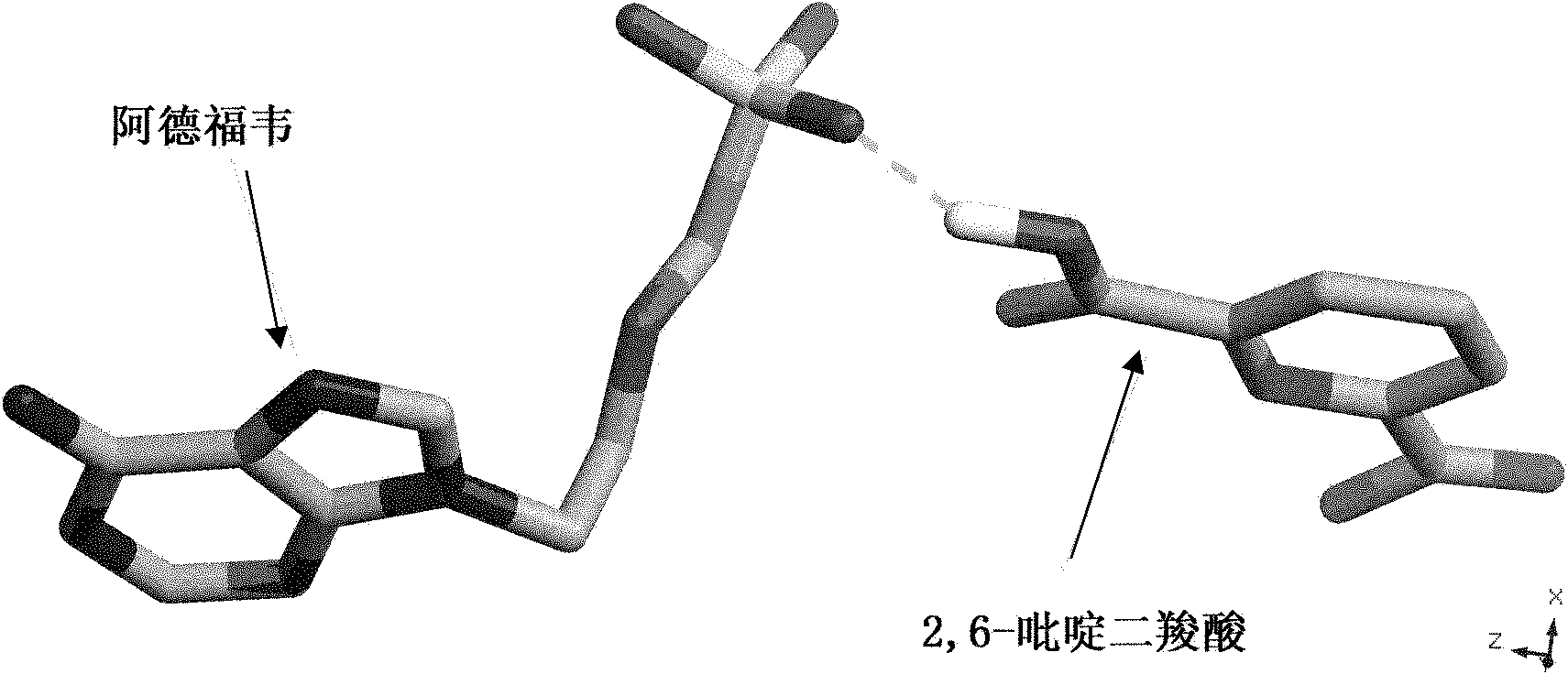
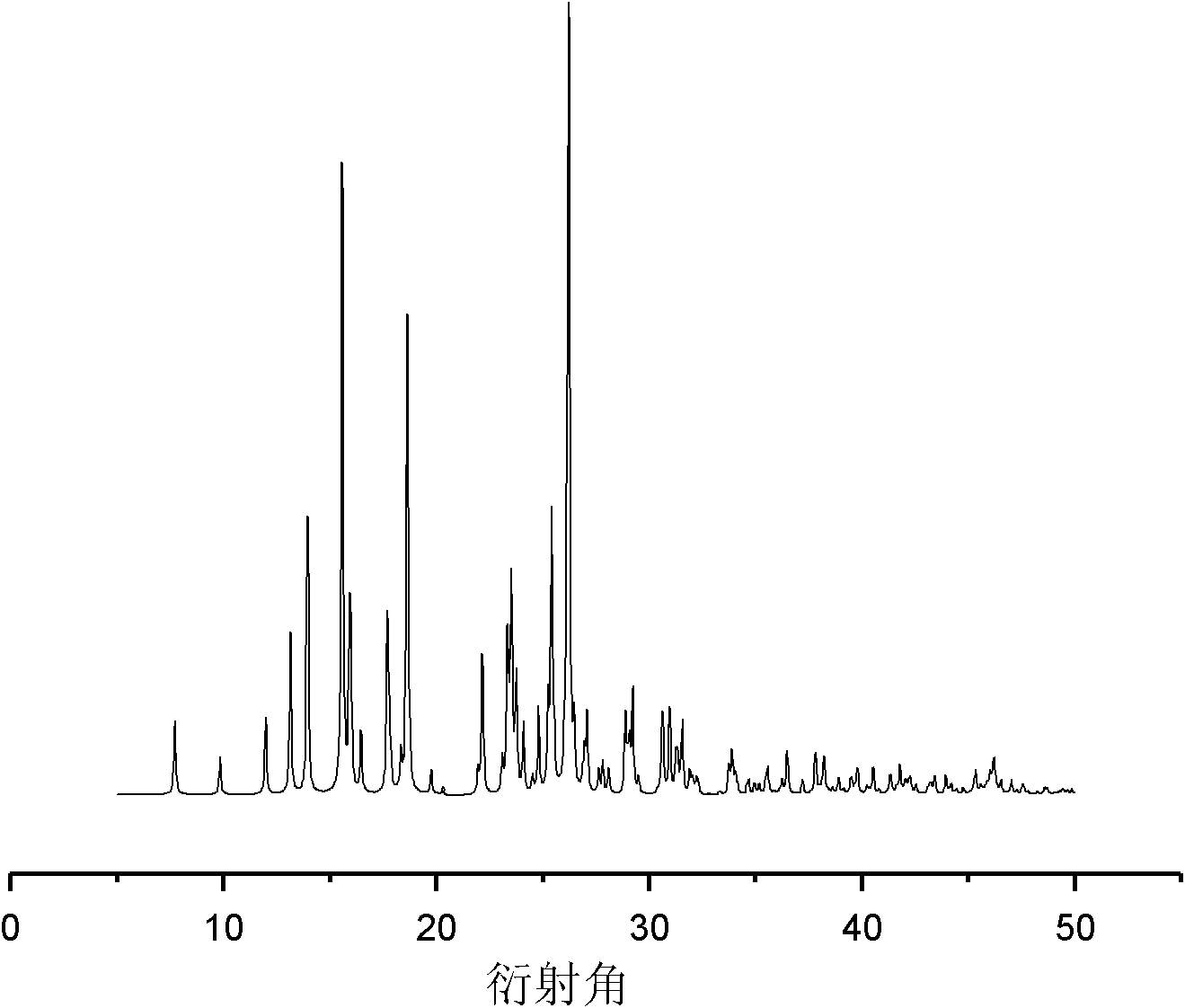
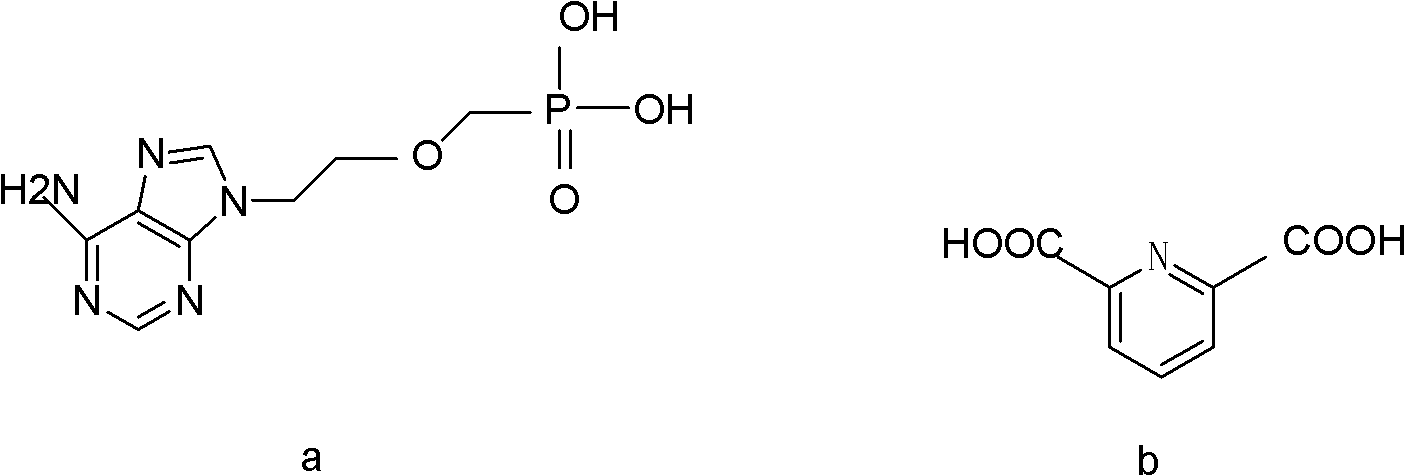
Adefovir pharmaceutical co-crystal and preparation method thereof
A drug and a combined technology, applied in the field of new adefovir drug co-crystal and the preparation of the drug co-crystal, to achieve the effect of improving stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of co-crystals using adefovir and 2,6-pyridinedicarboxylic acid:
[0026] Weighing:
[0027] The reactant is fed according to the mass ratio of adefovir:2,6-pyridinedicarboxylic acid=3:2. Accurately weigh 30.0 mg of adefovir and 20.0 mg of 2,6-pyridinedicarboxylic acid in a round bottom flask with an analytical balance;
[0028] Dissolution of API:
[0029] Accurately measure 2ml of CH with a 5ml pipette 3 CH 2 OH and 2ml H 2 O in a round-bottomed flask, then put a 1cm magnetic stirrer, set up a reflux device, raise the temperature of the reaction system to 90°C, stir and reflux for 3h.
[0030] Reflux-solvent room temperature volatilization heat method:
[0031] After refluxing for 3 hours, take out the stirring bar, filter, and place the filtrate in a transparent glass vial at room temperature. Afterwards, the solvent is slowly volatilized at room temperature, and transparent flaky crystals are formed after 30 hours, which is the adefovir drug eutecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com