Medical devices for use in the surgical treatment of hyperproliferative diseases affecting the spinal cord
A surgical and medical technique used in therapy, spinal cord nerve electrodes, electrotherapy, etc. to solve problems such as providing benefits to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
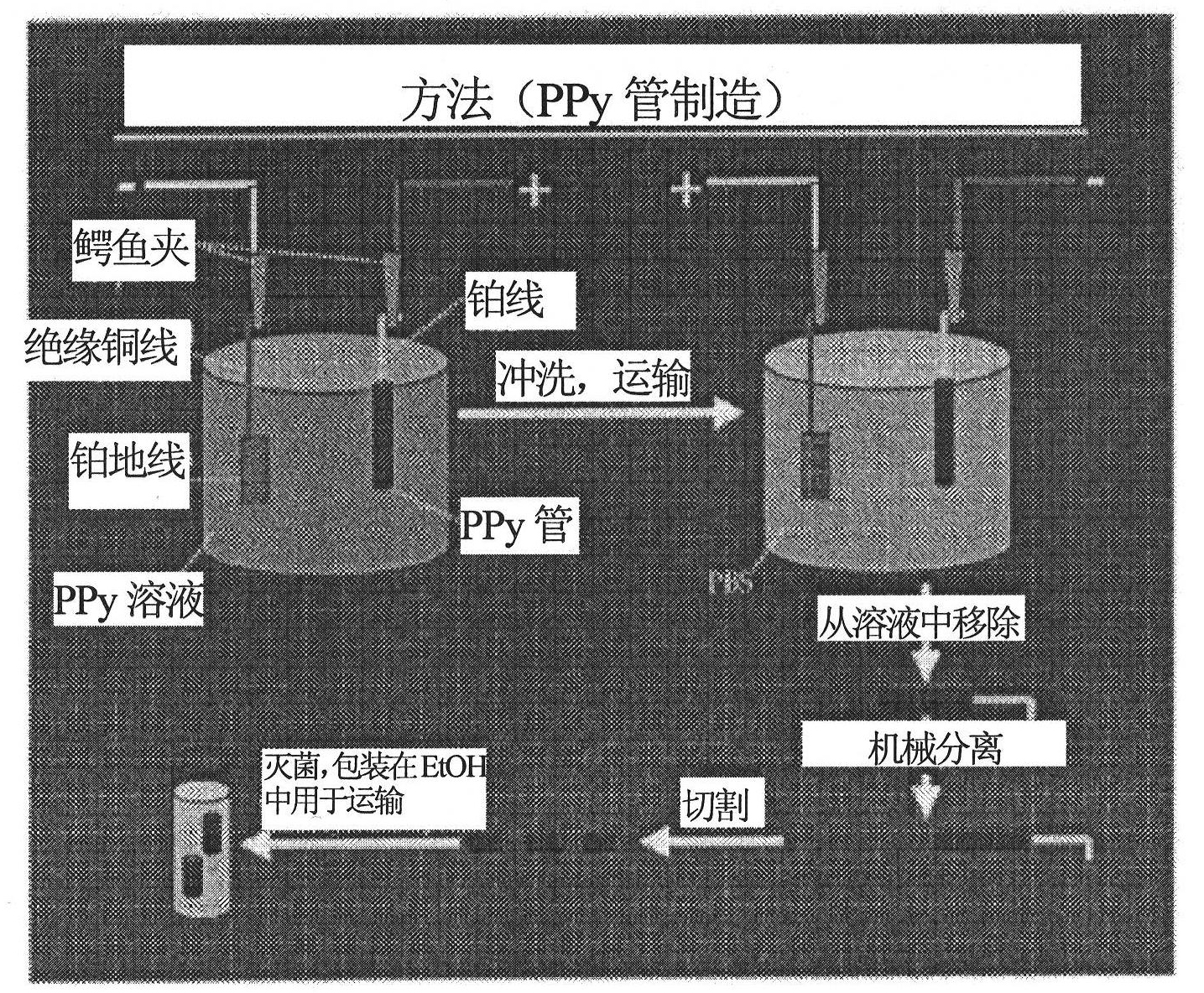
Method used
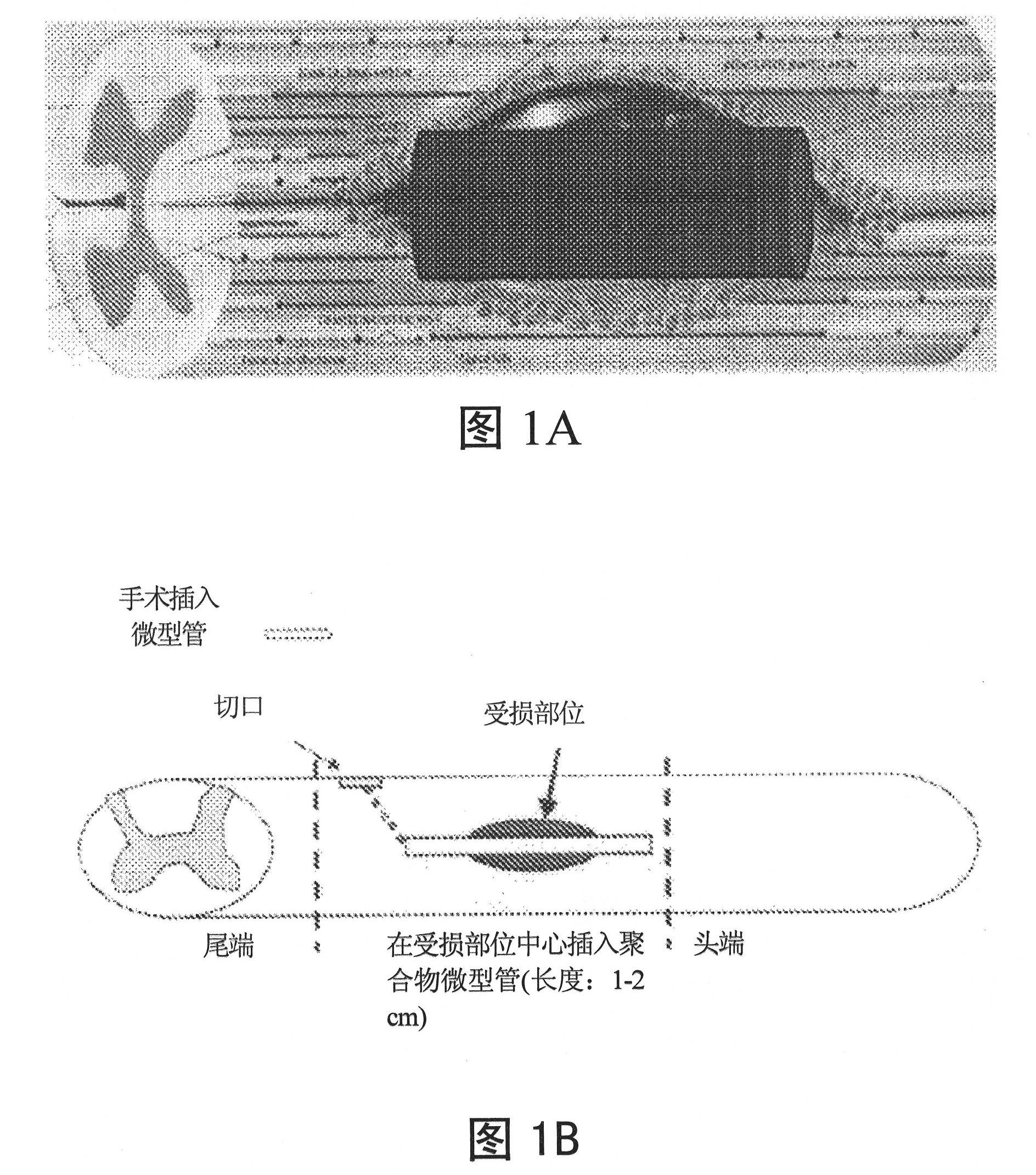
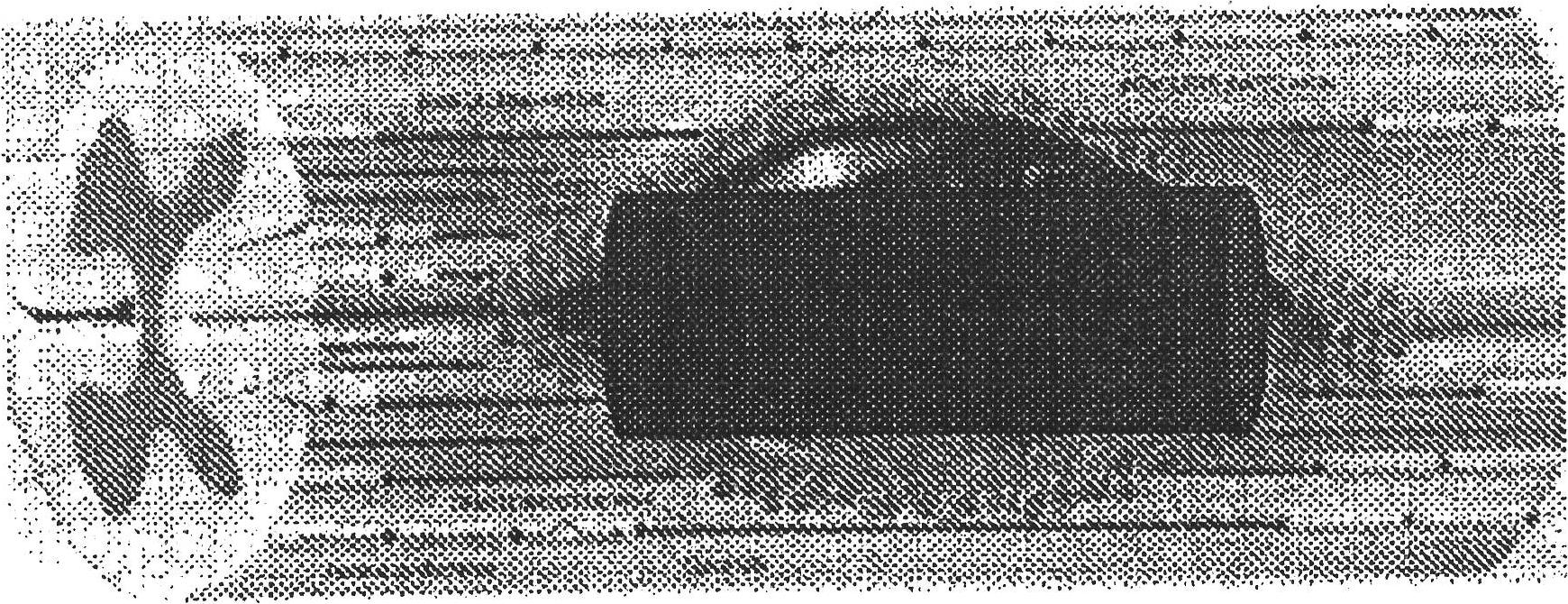
Image
Examples
Embodiment 1
[0089] Embodiment 1, polypyrrole microtube (mini-tube) manufacture (1)
[0090] Polypyrrole tube scaffolds were formed by electrodepositing erodible PPy onto 250 μm diameter platinum wires at 100 μA for 30 min. see for example figure 2 . After this step, reverse plating was performed at 3V for 5 minutes to enable removal of the scaffold. The current, timing, voltage and other parameters of this embodiment are not intended to be limiting.
Embodiment 2
[0091] The manufacture (II) of embodiment 2, PPy micropipe
[0092] Tubular PPy scaffolds were fabricated by electroplating PPy on conductive wire molds. This technique can be scaled to produce stents of arbitrary length, inner and outer diameter. Also, the plating temperature can be used (see figure 2 ) controls the surface roughness. Extraction of the scaffold from the template was achieved by applying a negative potential in saline solution. This negative potential causes electrochemical reduction and slightly increases the scaffold size. It can then be mechanically detached from the platinum wire mold with light application of force without damage to the material. This technique is an improvement over existing methods that use harsh organics to etch internal lines. For in vivo use, PPy tube scaffolds were formed by electrodeposition of erodible PPy onto platinum wires with a diameter of 250 μm at 100 μA for 40 min, followed by reverse plating at 3 V for 20 s, enablin...
Embodiment 3
[0093] Embodiment 3, single stent manufacture
[0094] A 50:50 poly(lactic-co-glycolic acid) (PLGA) (75%, number average molecular weight, Mn, about 40,000) and a block copolymer of poly(lactic-co-glycolic acid-polylysine) (25%, A mixture of PLGA block Mn about 30,000 and polylysine block Mn about 2000) is made into a single scaffold. PLGA was chosen to achieve a degradation rate of approximately 30-60 days, and functionalized polymers were incorporated to provide possible sites for surface modification. Single scaffolds were fabricated using the salt leaching method: a 5% (wt / vol) solution of the polymer mixture in chloroform was cast on salt in the diameter range of 250-500 μm and the solvent was allowed to evaporate. The salt is then soaked in water to form a single porous polymer layer that can be seeded with stem cells or other agents.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com