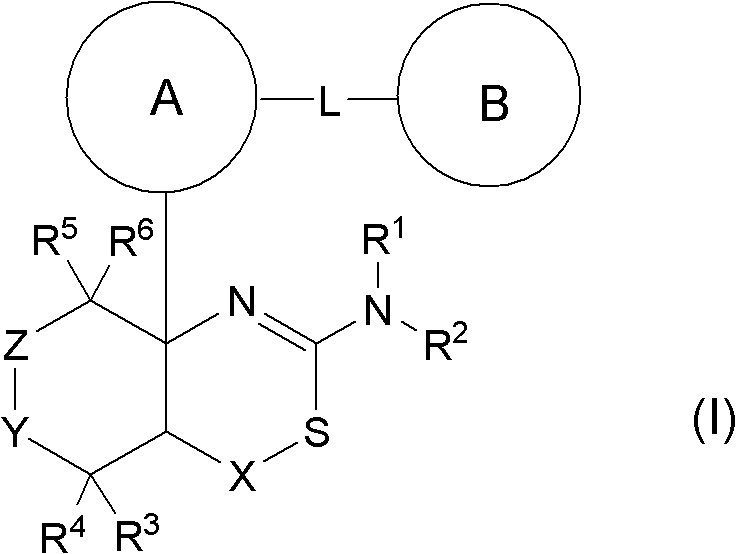
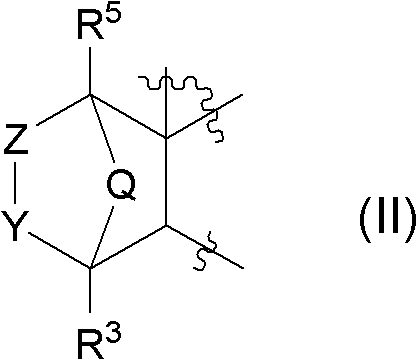
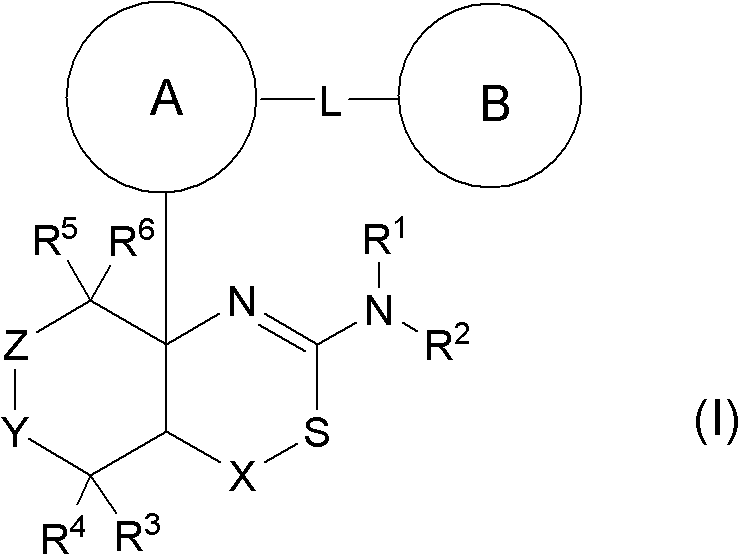
Novel fused aminodihydrothiazine derivative
A compound, alkyl technology, applied in the field of fused aminodihydrothiazine derivatives, can solve problems such as no fundamental treatment has been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0614] (-)-[(4aR * ,8aS * )-8a-(5-amino-2-fluorophenyl)-4,4a,5,6,8,8a-hexahydro-7-oxa-3-thia-1- Synthesis of tert-butyl azinaphthalen-2-yl]carbamate
[0615] [Formula 26]
[0616]
[0617] (1) Synthesis of 3-butenyloxyglyoxaldoxime
[0618] Hydroxylamine sulfate (20.5g), sodium acetate (12.8g) and water (20ml) were added to 3-butenyloxyacetaldehyde (17.8g; J.Chem.Soc., Perkin Trans.1, 1999, 3143-3155) in ethanol (200ml) solution. The mixture was stirred overnight at room temperature, then water was added. Excess ethanol was evaporated under reduced pressure and the resulting residue was extracted with ethyl acetate. The organic layer was washed with brine and dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure and the residue was purified by silica gel column chromatography to obtain the title compound (6.20 g).
[0619] 1 H-NMR (400MHz, CDCl 3 )δ (ppm): 2.36 (m, 2H), 3.53 (dt, J = 6.6, 8.4Hz, 2H), 4.10 (d, J = 5....
preparation Embodiment 2
[0648] Synthesis of 5-fluoromethoxypyrazine-2-carboxylic acid
[0649] [Formula 27]
[0650]
[0651] (1) Synthesis of Methyl 5-Fluoromethoxypyrazine-2-carboxylate
[0652] Toluene-4-sulfonic acid fluoromethyl ester (Journal of Labeled Compounds & Radiopharmaceuticals, 46(6), 555-566; 2003) (344 mg) and cesium carbonate (824 mg) were added to 5-hydroxypyrazine-2-carboxylic acid methyl ester (130mg) in N,N-dimethylformamide (2.0mL). The reaction solution was stirred at 70°C for 5 hours and 30 minutes, then cooled to room temperature. Water was added to the reaction solution, followed by extraction with ethyl acetate. The organic layer was concentrated under reduced pressure. The residue was purified by NH-silica gel column chromatography to obtain the title compound (18.0 mg).
[0653] 1 H-NMR (400MHz, CDCl 3 ) δ (ppm): 4.03 (s, 3H), 6.14 (d, J=51.2Hz, 2H), 8.42 (d, J=1.2Hz, 1H), 8.94 (d, J=1.2Hz, 1H).
[0654] (2) Synthesis of 5-fluoromethoxypyrazine-2-carboxy...
preparation Embodiment 3
[0658] Synthesis of 5-cyanopyridine-2-carboxylic acid
[0659] [Formula 28]
[0660]
[0661] (1) Synthesis of methyl 5-cyanopyridine-2-carboxylate
[0662] A mixture of methyl 5-bromopyridine-2-carboxylate (2.8 g) and copper cyanide (3.6 g) in NMP (30 ml) was heated at 170° C. for 1.5 hours with stirring. Water was added to the reaction solution at room temperature and insolubles were removed by filtration. The filtrate was extracted with ethyl acetate. The extract was washed with brine, and dried over anhydrous magnesium sulfate. The drying agent was filtered off and the filtrate was concentrated under reduced pressure. The resulting crude product was purified by silica gel column chromatography to obtain the title compound (920 mg).
[0663] 1 H-NMR (400MHz, CDCl 3 )δ(ppm): 4.06(s, 3H), 8.16(dd, J=2.0, 8.0Hz, 1H), 8.27(d, J=8.0Hz, 1H), 9.01(d, J=2.0Hz, 1H) .
[0664] (2) Synthesis of 5-cyanopyridine-2-carboxylic acid
[0665] A solution of the compound (9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com