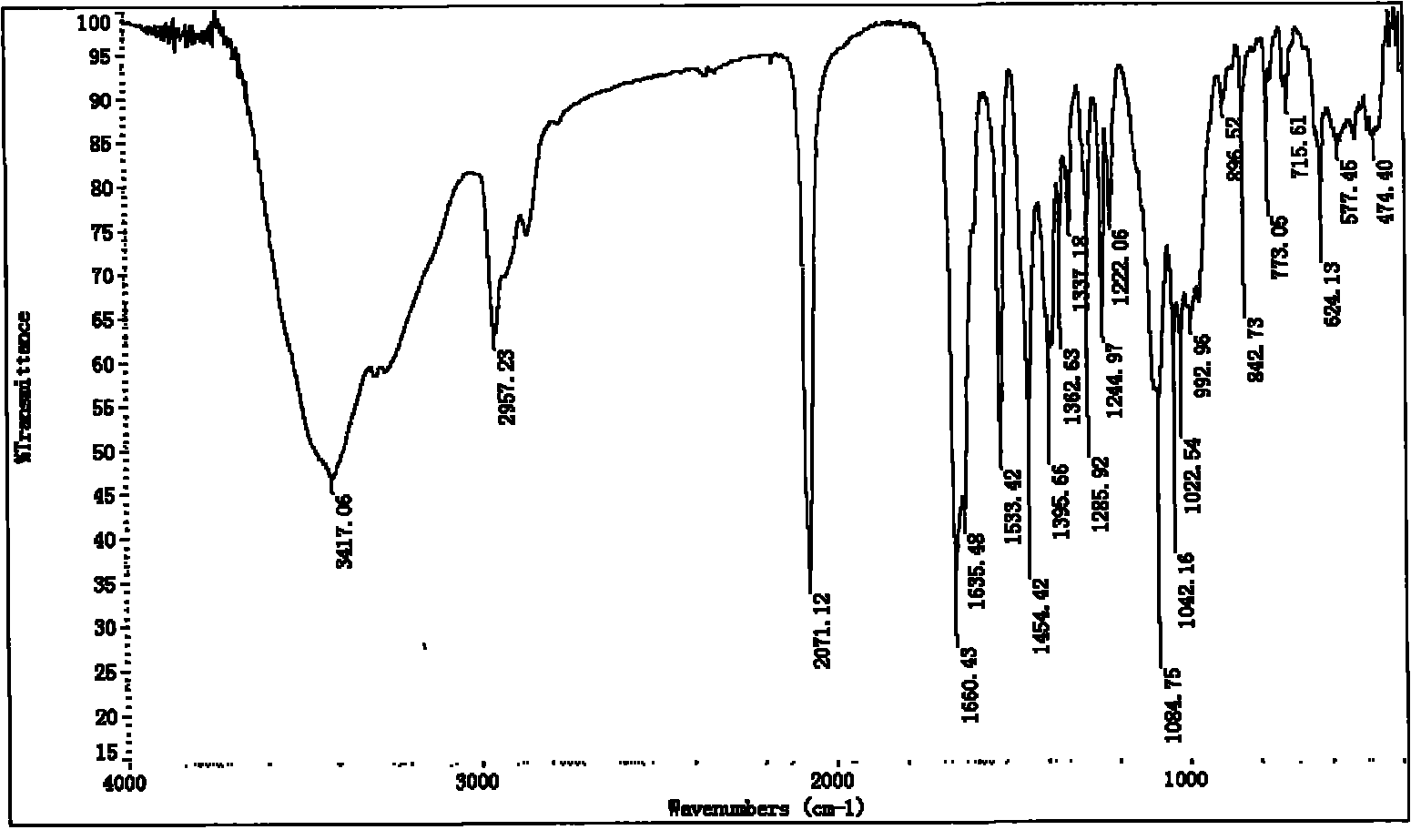
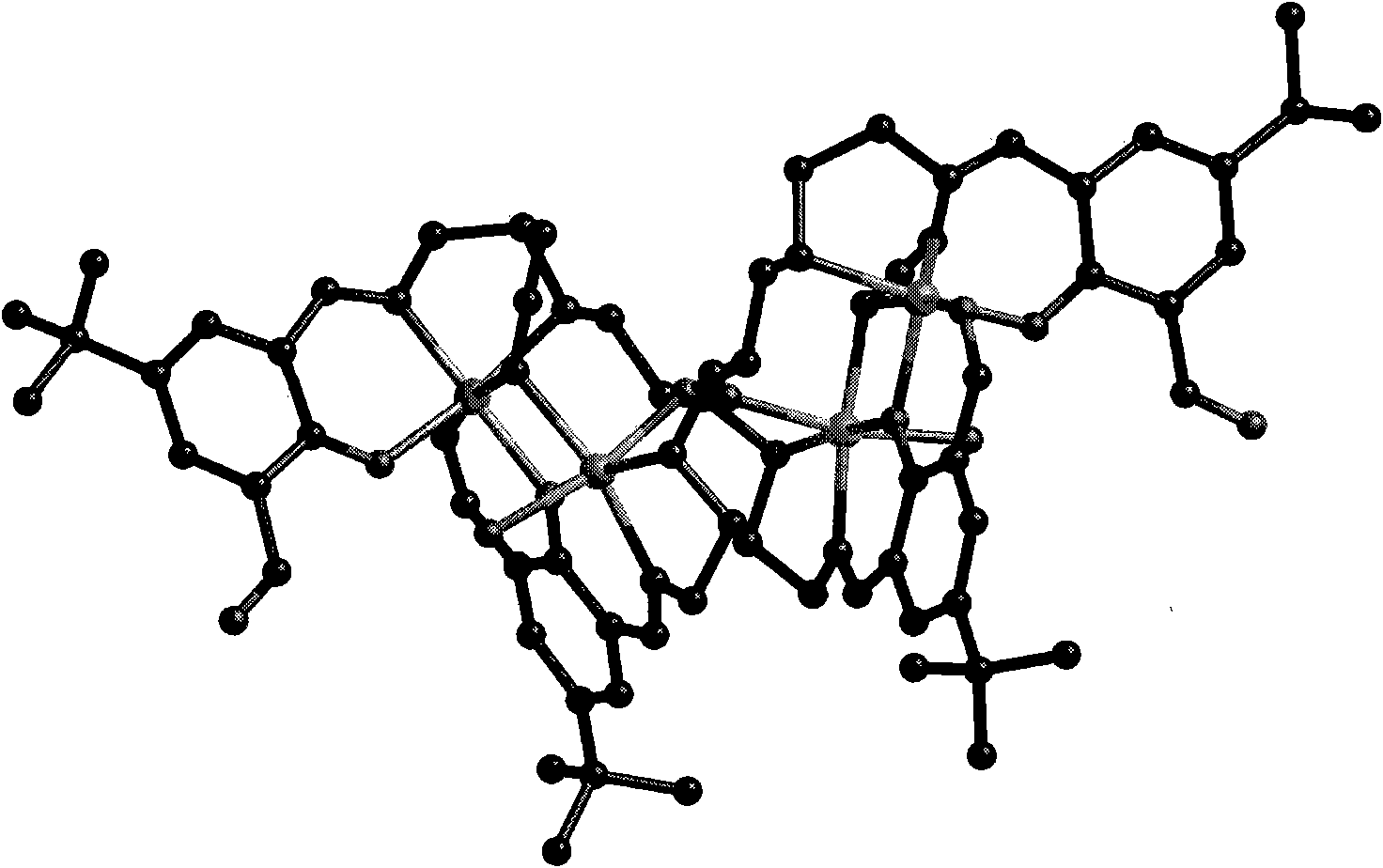
Differently substituted 2-hydroxy-1,3-phthalaldehyde and long-chain dual-amine mixed nickel complex and preparation method thereof
A technology of phthalaldehyde and nickel complexes, applied in the direction of nickel organic compounds, etc., can solve the problems of difficult purification and separation of samples, high risk and high temperature, and achieve the effects of high yield, overcoming high temperature and high pressure, and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 complex
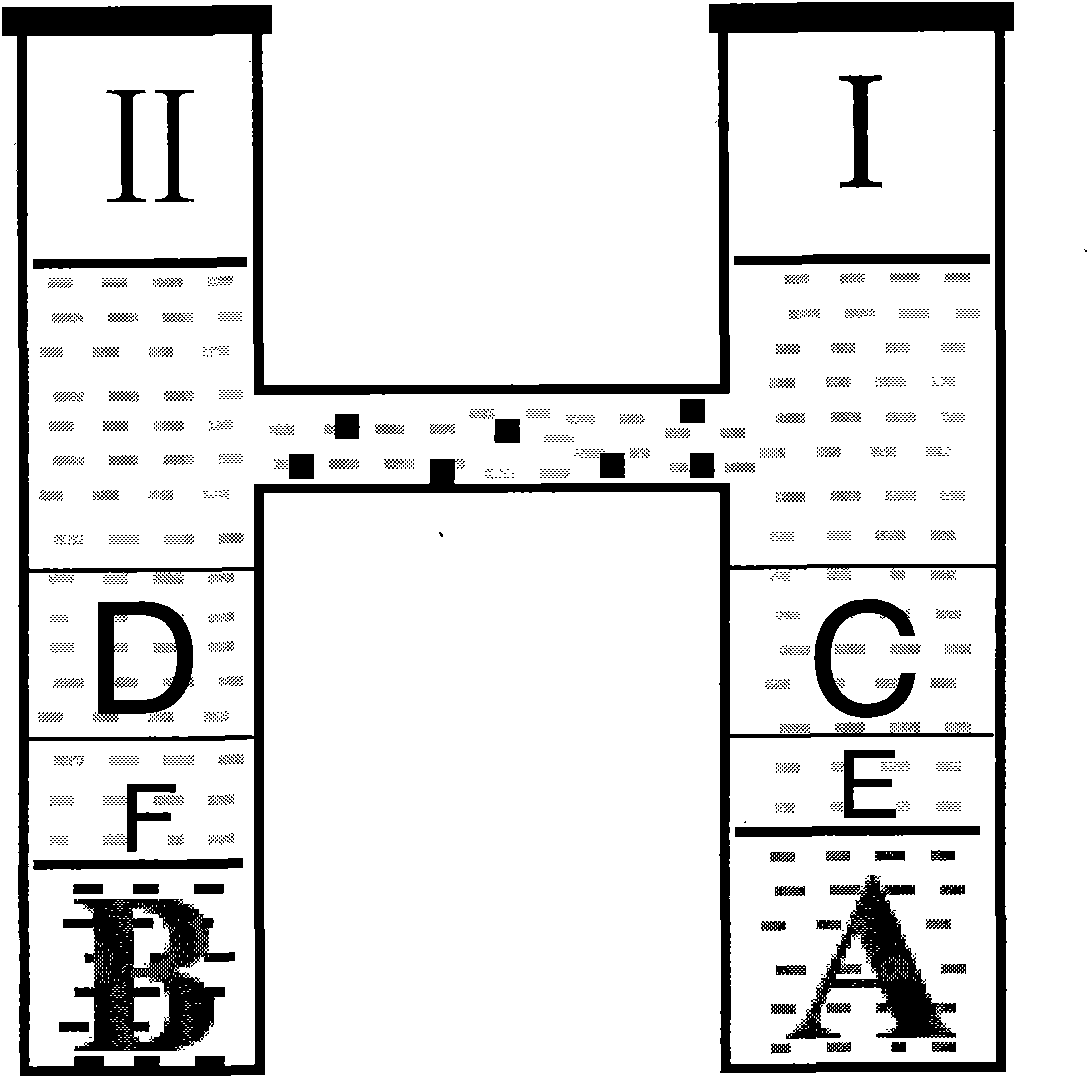
[0023] Put 0.1mmol 5-isopropyl-2-hydroxyl-1,3-benzenedicarbaldehyde on the bottom layer of the first side of the H-type tube, add a small amount of distilled water to dissolve to form reaction solution A, add methanol and water volume to the upper layer of reaction solution A 2mL of mixed solution E with a ratio of 1:1, add 0.3mmol of 5-tert-butyl-2-hydroxyl-1,3-benzenedicarbaldehyde and 5-isopropyl-2-hydroxyl-1 to the upper layer of mixed solution E, Methanol solution C of 3-phthalaldehyde; add 0.4mmol Ni(ClO 4 ) 2 ·6H 2 O, 0.2 mmol NaN 3 , Dissolved with 5mL of distilled water to form reactant B, add 2mL of mixed solution F of methanol and water with a volume ratio of 1:1 in the upper layer of reaction solution B; then add 0.2mmol 1,3-diaminoethylamino-2 in the upper layer of solution F -Hydroxypropane, dissolved in 5mL of methanol to form reaction solution D, then slowly drop methanol into the solution until the two sides...
Embodiment 2
[0025] The preparation of embodiment 2 complexes
[0026] Put 0.1mmol 5-isopropyl-2-hydroxyl-1,3-benzenedicarbaldehyde on the bottom layer of the first side of the H-type tube, add a small amount of distilled water to dissolve to form reaction solution A, add methanol and water volume to the upper layer of reaction solution A 2mL of mixed solution E with a ratio of 1:1, add 0.3mmol of 5-tert-butyl-2-hydroxyl-1,3-benzenedicarbaldehyde and 5-isopropyl-2-hydroxyl-1 to the upper layer of mixed solution E, Methanol solution C of 3-phthalaldehyde; add 0.4mmol Ni(AcO) to the bottom layer of the II side of the H-tube 2 4H 2 O, 0.2 mmol NaN 3 , Dissolved with 5mL of distilled water to form reactant B, add 2mL of mixed solution F of methanol and water with a volume ratio of 1:1 in the upper layer of reaction solution B; then add 0.2mmol 1,3-diaminoethylamino-2 in the upper layer of solution F -Hydroxypropane, dissolved in 5mL of methanol to form reaction solution D, then slowly drop ...
Embodiment 3
[0028] The preparation of embodiment 3 complexes
[0029] Put 0.1mmol 5-isopropyl-2-hydroxyl-1,3-benzenedicarbaldehyde on the bottom layer of the first side of the H-type tube, add a small amount of distilled water to dissolve to form reaction solution A, add methanol and water volume to the upper layer of reaction solution A 2mL of mixed solution E with a ratio of 1:1, add 0.3mmol of 5-tert-butyl-2-hydroxyl-1,3-benzenedicarbaldehyde and 5-isopropyl-2-hydroxyl-1 to the upper layer of mixed solution E, Methanol solution C of 3-phthalaldehyde; add 0.4mmol NiCl to the bottom layer of the II side of the H-tube 2 ·6H2 O, 0.2 mmol NaN 3 , Dissolved with 5mL of distilled water to form reactant B, add 2mL of mixed solution F of methanol and water with a volume ratio of 1:1 in the upper layer of reaction solution B; then add 0.2mmol 1,3-diaminoethylamino-2 in the upper layer of solution F -Hydroxypropane, dissolved in 5mL of methanol to form reaction solution D, then slowly drop meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com