Method for preparing exenatide
A technology of exenatide and peptide resin, applied in the field of peptide solid-phase synthesis, can solve the problems of difficult stability, many control items, and many by-products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
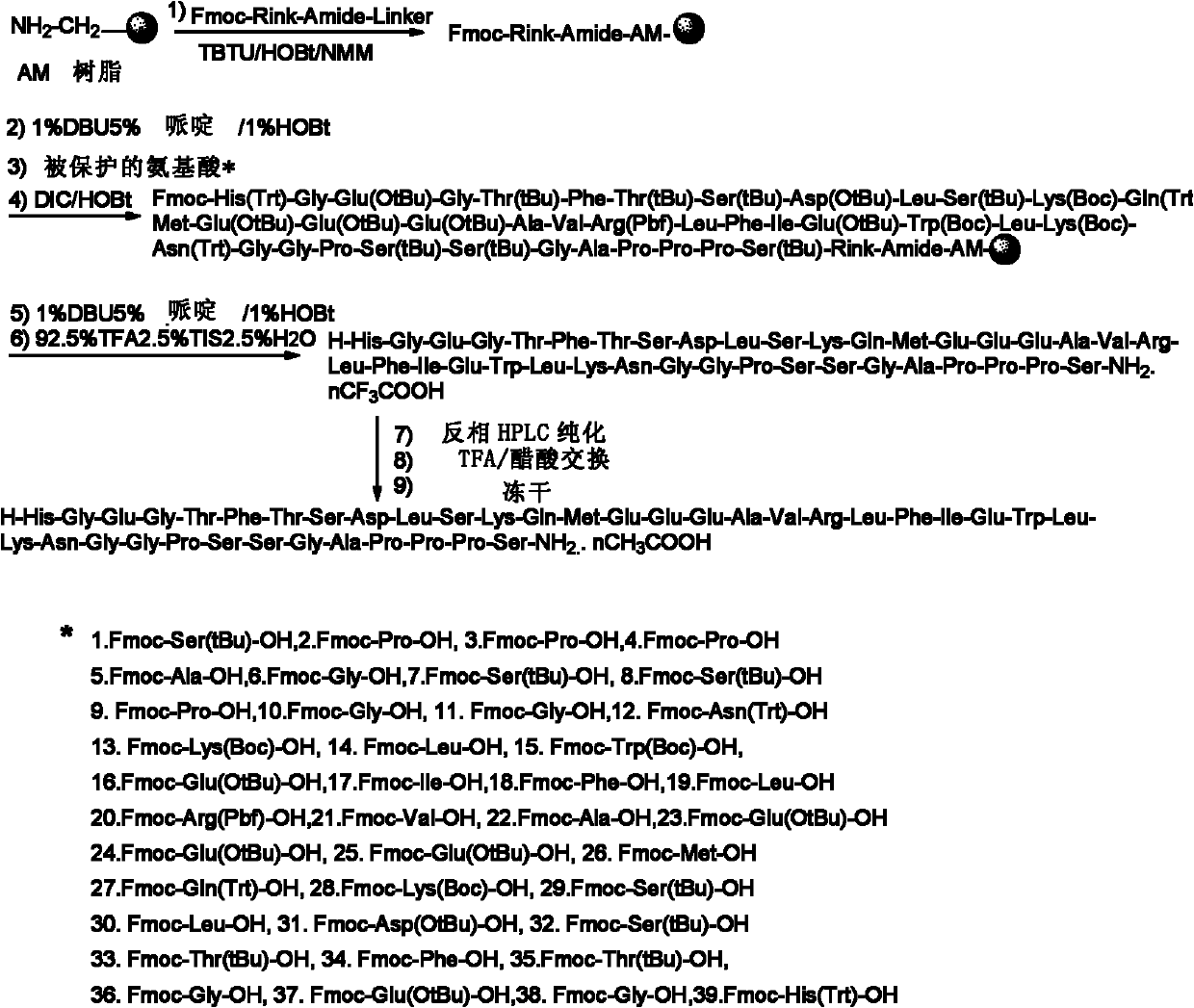
[0031] In one example of the present invention, the preparation method of solid-phase synthesis of exenatide by the polypeptide of the present invention comprises the following steps:
[0032] In the first step, AM polystyrene resin, Fmoc-Rink-Amide-Linker, TBTU, HOBt, and NMM are mixed to obtain Fmoc-Rink amide AM resin, whose degree of substitution is 0.4-1.6mmol / g;
[0033] In the second step, the deprotecting agent of the present invention is mixed with the Fmoc-Rink amide AM resin to remove the Fmoc group to obtain the Rink amide AM resin;
[0034] The third step is to condense Fmoc-Ser(tBu)-OH and Rink amide AM resin to obtain Fmoc-Ser(tBu)-Rink amide AM resin;
[0035] The fourth step, using the deprotecting agent of the present invention to remove the Fmoc group;
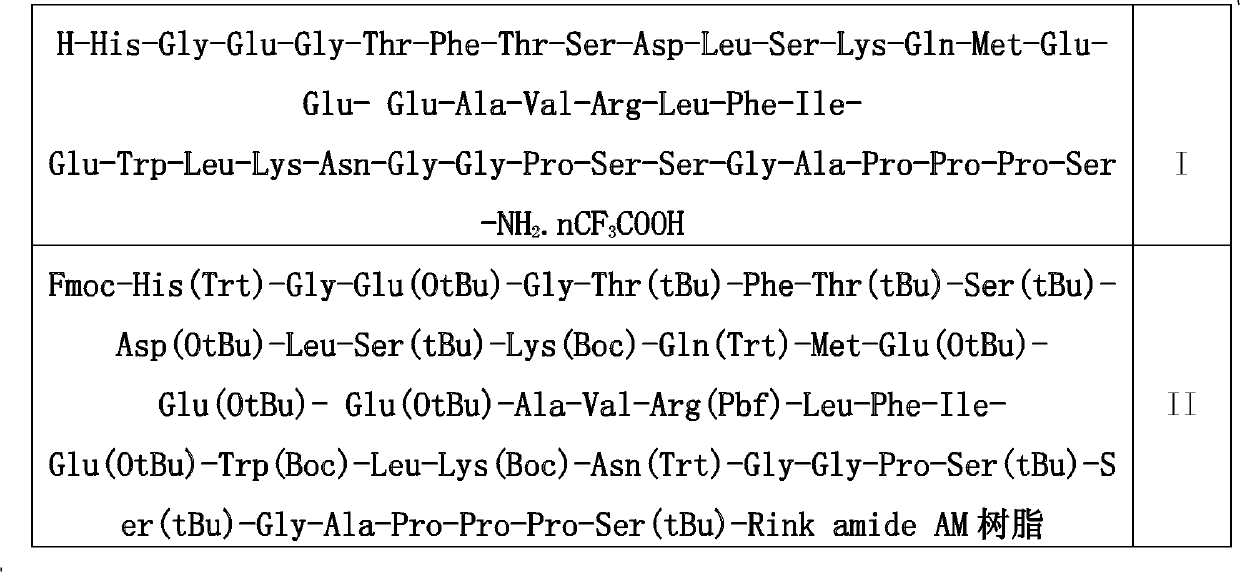
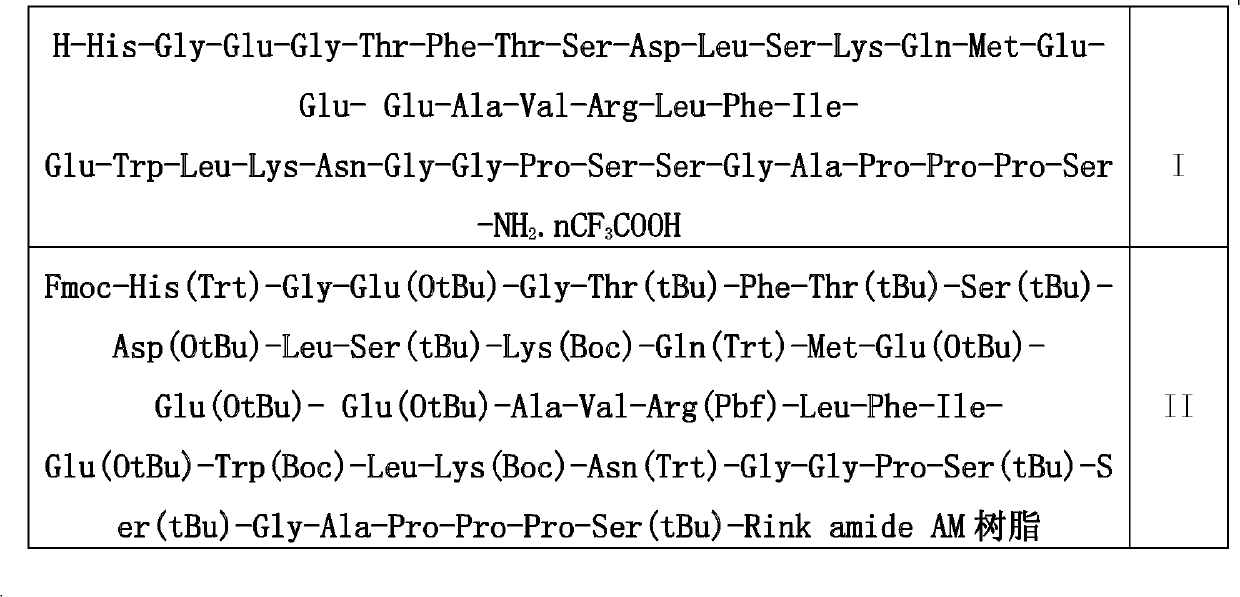
[0036] In the fifth step, the above-mentioned peptide bond formation steps are repeated to make the peptide chain grow from the C-terminus to the N-terminus until Fmoc-His(Trt)-Gly-Glu(OtBu)-Gly-Thr(tBu)-Ph...
Embodiment 1
[0061] Load Fmoc-Rink Amide Linker
[0062] Use 24.0g of AM polystyrene resin (substitution degree 0.6-0.9mmole / g) and 1.0 equivalent of Fmoc-Rink-Amide-Linker, 1.425 equivalent of TBTU, 1.5 equivalent of HOBT and 3 equivalents of NMM to react with stirring for 3 hours, and the reaction on the resin is not complete The amino group was capped with Ac2O / Pyridine / DMF (v / v / v), and finally the obtained resin was 34g, and the degree of substitution was 0.58mmole / g.
[0063] Deprotection
[0064] 8%piperidine / 1.5DBU / 5%HOBt / DMF (v / v / w / v) was used to deprotect twice consecutively, and the time was 10min and 20min respectively. Wash with DMF and methanol, respectively. After exhaustion, Kaiser test was used to evaluate the removal of Fmoc.
[0065] amino acid condensation
[0066] Add Fmoc-AA-OH / HOBt (1.0 equivalent / 1.0 equivalent) to the reactor, 1.0 equivalent relative to Fmoc-Rinkamide AM resin) / DMF solution, then add DIC (1.5 equivalent relative to Fmoc-Rinkamide AM resin), stir...
Embodiment 2
[0077] Load Fmoc-Rink Amide Linker
[0078] Use 24.0g of AM polystyrene resin (substitution degree 0.8-1.0mmole / g) and 1.5 equivalents of Fmoc-Rink-Amide-Linker, 1.425 equivalents of TBTU, 1.5 equivalents of HOBT and 3 equivalents of NMM to stir for 3 hours, and the reaction on the resin is not complete The amino group was capped with Ac2O / Pyridine / DMF, and finally the obtained resin was 34g, and the degree of substitution was 0.60mmole / g.
[0079] Deprotection
[0080] Use 6%piperidine / 1.3DBU / 2%HOBt / DMF (v / v / w / v) for two consecutive deprotection times for 10min and 20min respectively. Wash with DMF and methanol, respectively. After exhaustion, Kaiser test was used to evaluate the removal of Fmoc.
[0081] amino acid condensation
[0082] Add Fmoc-AA-OH / HOBt (1.5 equivalents / 1.5 equivalents) to the reactor, 1.5 equivalents relative to Fmoc-Rinkamide AM resin) / DMF solution, then add DIC (2.0 equivalents relative to Fmoc-Rinkamide AM resin), stir After 45 minutes, DIC (2.0 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com