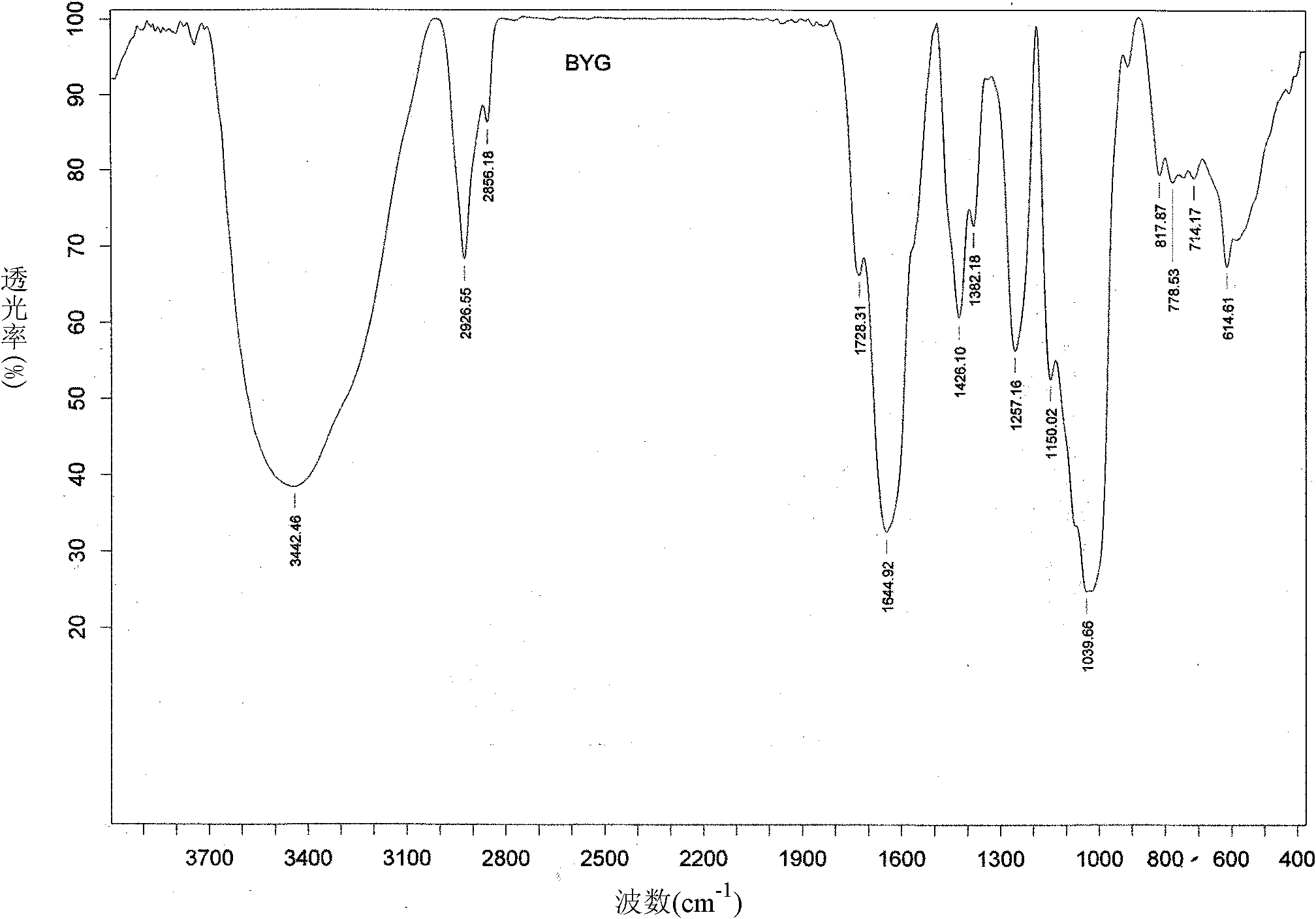
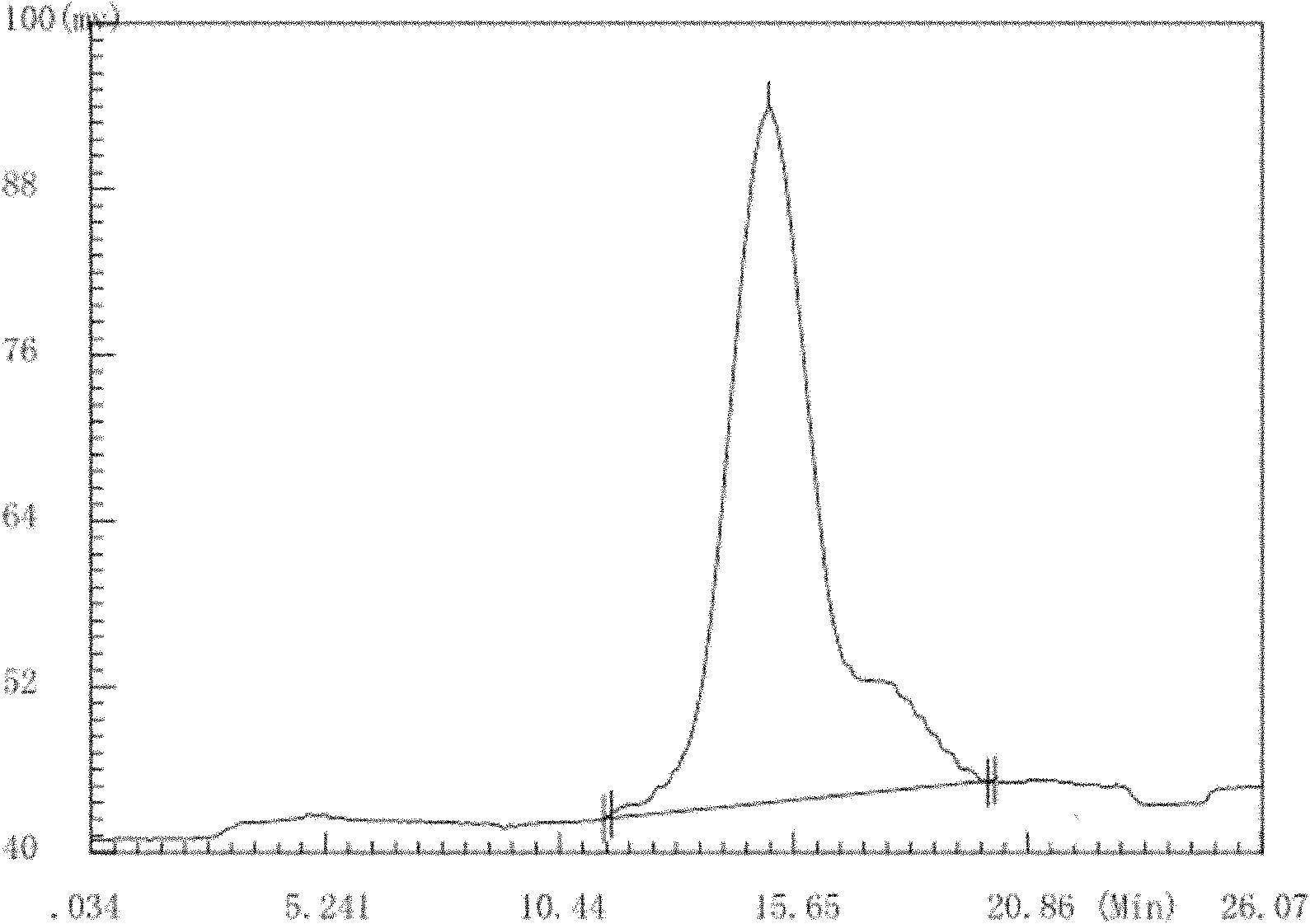
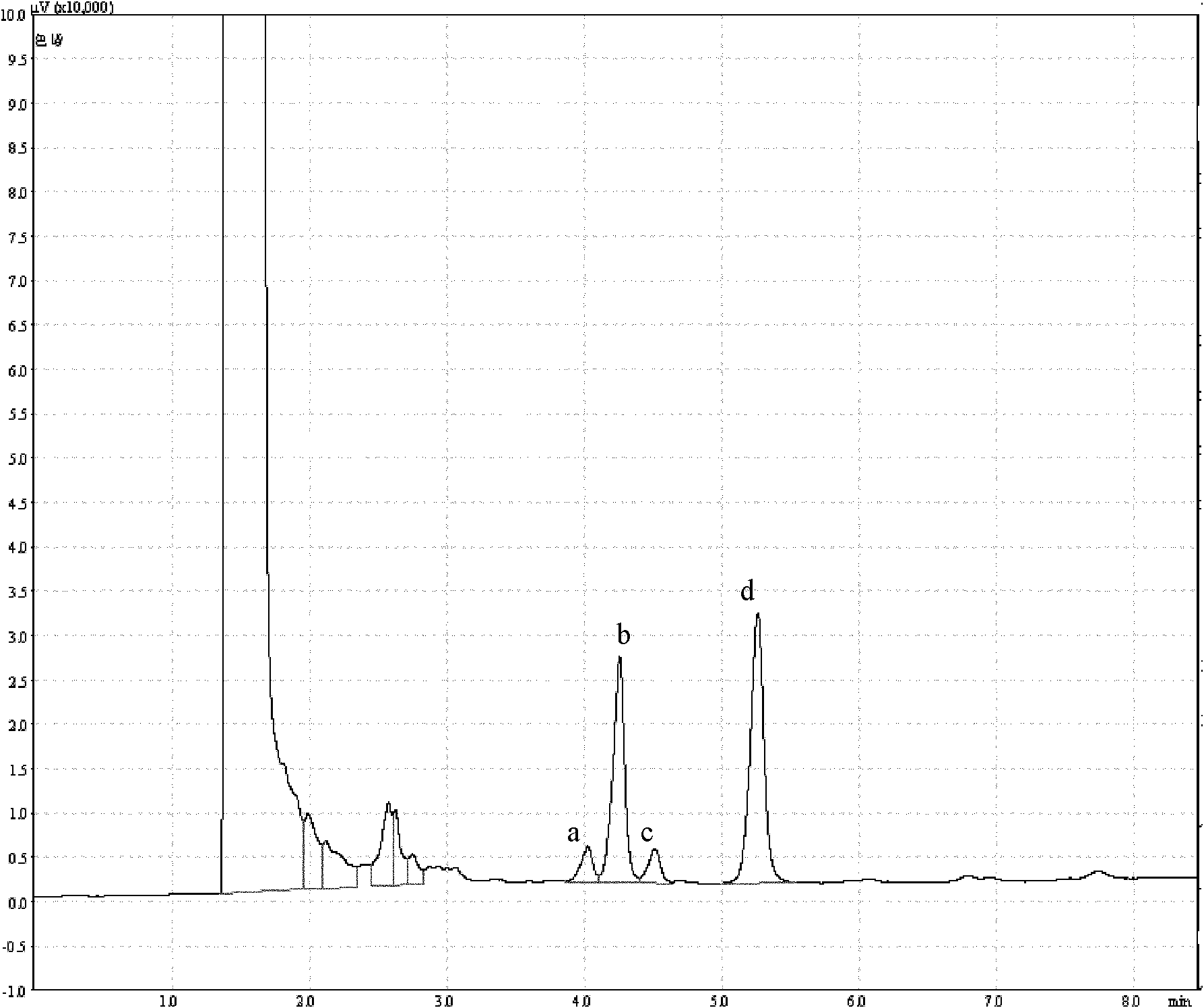
Antarctic cold-adapted microbial extracellular polysaccharide capable of improving body immunity
A technology of extracellular polysaccharides and immunity, applied in the field of exopolysaccharides of cold-adapted microorganisms in Antarctica
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A method for preparing an exopolysaccharide of cold-adapted microorganisms in Antarctica capable of improving body immunity, comprising the steps of:
[0033] (1) After activating the Antarctic cold-adapted bacterium (Pseudoaltermonnas sp.) S-5 with the strain preservation number CGMCC NO.3433, inoculate it in Zobell 2216E liquid medium, cultivate it at 9°C for 24 hours, and inoculate it at 1 wt%. Zobell 2216E liquid medium is used to expand the culture, and the bacteria can be multiplied;
[0034] (2) by the inoculum amount of 10wt% by the multiplication bacterial classification that step (1) makes, be inoculated in the fermentation medium in the liquid fermentation tank, after adding the sterilized soybean oil of fermentation medium weight 0.3% in the fermentation medium , 7-9°C aerobic stirring culture for 50-60h, stirring speed 160-170r / min, to obtain a liquid fermentation broth;
[0035] (3) Centrifuge the liquid fermentation broth prepared in step (2) for 15min a...
Embodiment 2
[0045] Example 2 Effect of in vitro administration of exopolysaccharides from cold-adapted microorganisms on the active proliferation of mouse spleen lymphocytes
[0046] The mice were sacrificed by cervical dislocation, and the spleen was taken aseptically to prepare splenic lymphocytes, which were made into 1×10 6 / ml cell suspension. Mouse 1 x 10 6 / ml splenocyte suspension was added to a 96-well cell culture plate, 100 μl per well, and the experiment was set up as a control group, exopolysaccharide (final concentrations were 0.1, 0.25, 0.5, 1, 2.5, 5, 10 μg / ml) stimulation group, For ConA (final concentration: 5 μg / ml) stimulation group, six replicate wells were set up for each concentration in each group, and the blank control group was replaced by an equal volume of RPMI 1640. At 37°C, 5% CO 2 Cultivate for 72 hours in a saturated humidity incubator. 10 μl of MTT solution was added to each well 4 hours before the end of the culture, and the culture was continued unti...
Embodiment 3
[0052] Example 3: Influence of in vitro administration of exopolysaccharides from cold-adapted microorganisms on the activity of acid phosphatase in mouse macrophages
[0053] Mice were intraperitoneally injected with 1ml of 4% starch solution, and sacrificed by cervical dislocation 3 days later, aseptically collected peritoneal macrophages of mice to prepare cell suspension; the peritoneal cell suspension was added to a 96-well cell culture plate, 100 μl per well. At 37°C, 5% CO 2 CO 2 Incubate for 4 hours in the incubator. Remove the supernatant, and the adherent cells are macrophages. The experiment set up control group, exopolysaccharide (0.1, 0.25, 0.5, 1, 2.5, 5, 10 μg / ml final concentration) stimulation group, LPS (1 μg / ml final concentration) stimulation group, 100 μl per hole, each group Six replicate wells were set up for each concentration. At 37°C, 5% CO 2 CO 2 Incubate for 24 hours in the incubator. Take it out, remove the supernatant, add 100 μl of pNPP so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com