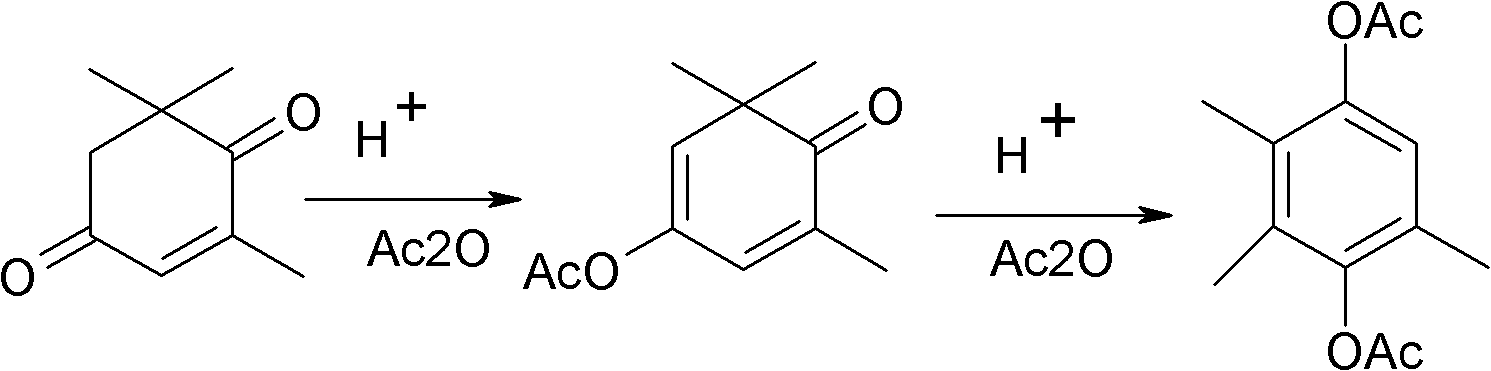
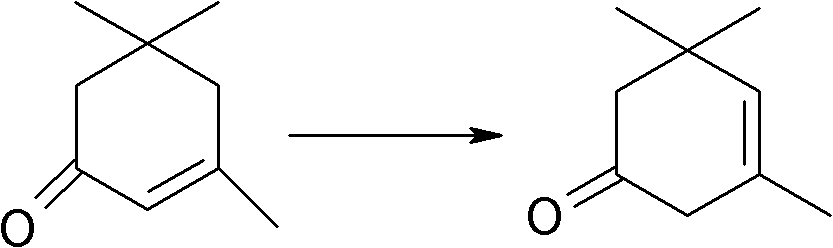
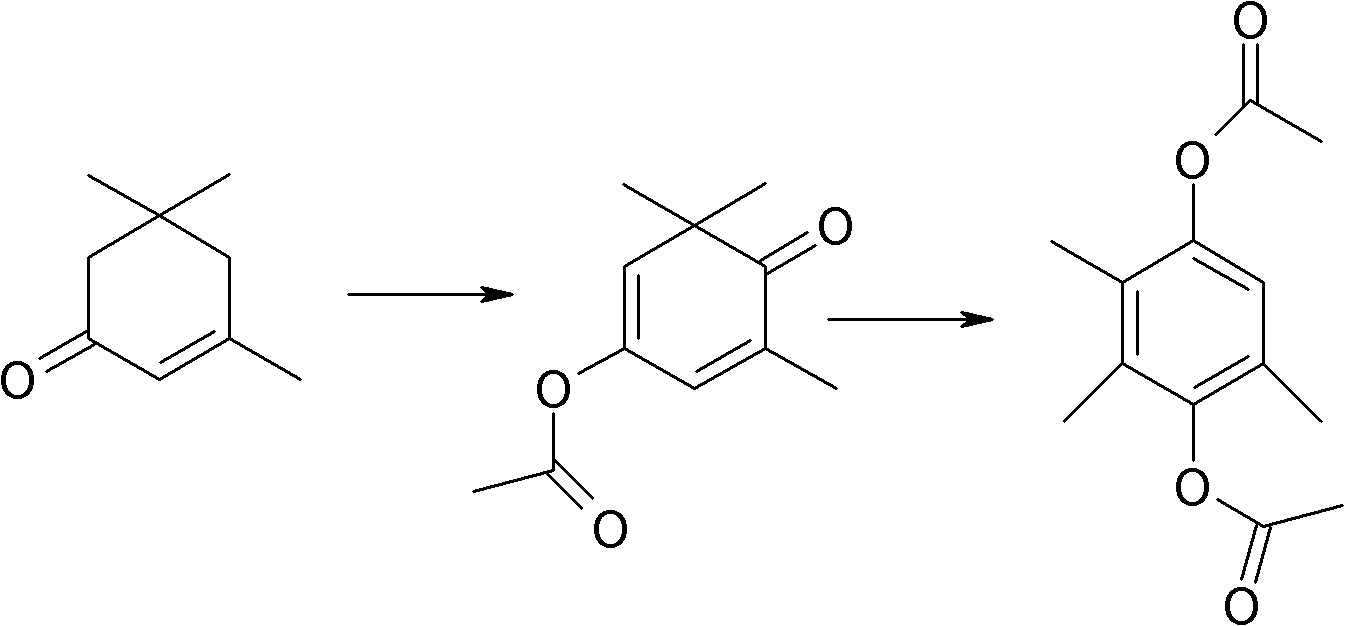
Method for synthesizing 2,3,5-trimethylhydroquinone diester
A technology of trimethylhydroquinone diester and synthetic method, which is applied in two fields, can solve the problems of high price of nitroxide free radical peroxide and unfavorable industrial production, and achieve low production cost, easy operation and high reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 1) Mix 1mol α-isophorone (138 grams) and 1.5mol (153 grams) acetic anhydride, add H 3 PMo 12 o 40 0.0043mol (7.85g), react at 100°C for 8 hours, recover the dry solvent, add 0.006mol (0.672g) of potassium tert-butoxide, DMSO 200ml, heat the oil bath to 115°C and feed air, react for 100h, and take a sample to detect that the raw material content is ≤1 %, stop feeding the air, spin the reaction solution to dry the solvent, and obtain the crude oil of the monoesterification product of ketoisophorone.
[0039] 2) Add 0.051mol (6.89g) HBr (60%) and 2mol (204g) acetic anhydride, cool to 0°C, add dropwise 0.004mol (0.9g) ZnBr dissolved in 100ml acetic acid 2 , control the dropping time for 5 hours, keep the reaction at 10°C for 20 hours after dropping, detect the content of intermediates ≤ 0.5%, recover excess acetic anhydride and acetic acid generated by the reaction, and crystallize the crude oil with acetic acid / water mixed solvent to obtain off-white or light 165 g of y...
Embodiment 2
[0041] 1) Mix 1mol α-isophorone (138 grams) and 1.5mol (153 grams) acetic anhydride, add H 3PMo 12 o 40 0.0043mol (7.85g), react at 100°C for 8 hours, recover the dry solvent, add 0.006mol (0.672g) of potassium tert-butoxide, DMSO 200ml, heat the oil bath to 150°C and feed air, react for 60h, and take a sample to detect that the raw material content is ≤1 %, stop feeding the air, spin the reaction solution to dry the solvent, and obtain the crude oil of the monoesterification product of ketoisophorone.
[0042] 2) Add 0.051mol (6.89g) HBr (60%) and 2mol (204g) acetic anhydride, cool to 0°C, add dropwise 0.004mol (0.9g) ZnBr dissolved in 100ml acetic acid 2 , control the dropping time for 5 hours, keep the reaction at 10°C for 20 hours after dropping, detect the content of intermediates ≤ 0.5%, recover excess acetic anhydride and acetic acid generated by the reaction, and crystallize the crude oil with acetic acid / water mixed solvent to obtain off-white or light 152g of yell...
Embodiment 3
[0044] 1) Mix 1mol α-isophorone (138 grams) and 1.5mol (153 grams) acetic anhydride, add H 3 PMo 12 o 40 0.0043mol (7.85g), react at 100°C for 8 hours, recover the dry solvent, add 0.006mol (0.672g) of potassium tert-butoxide, DMSO 200ml, heat the oil bath to 115°C and feed air, react for 24h, stop feeding air, and The reaction solution was spun to dry the solvent to obtain the crude oil of the monoesterification product of ketoisophorone.
[0045] 2) Add 0.051mol (6.89g) HBr (60%) and 2mol (204g) acetic anhydride, cool to 0°C, add dropwise 0.004mol (0.9g) ZnBr dissolved in 100ml acetic acid 2 , control the dropping time for 5 hours, keep the reaction at 10°C for 20 hours after dropping, detect the content of intermediates ≤ 0.5%, recover excess acetic anhydride and acetic acid generated by the reaction, and crystallize the crude oil with acetic acid / water mixed solvent to obtain off-white or light 105g of yellow crystals, internal standard 94.3%, mp: 109-110°C, yield 42.0%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com