Secnidazole derivative as well as preparation method and application thereof
A technology of secnidazole and derivatives, which is applied in the field of secnidazole derivatives and can solve problems such as unseen secnidazole and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 intermediate
[0025] (1) Preparation of methyl salicylate derivative II
[0026] Weigh salicylic acid derivative I (0.20mol) and add 160mL methanol, carefully add 18mL concentrated sulfuric acid dropwise under stirring, and heat to reflux for 15 hours. Stop heating, evaporate most of the methanol, and carefully add 20% Na 2 CO 3 Aqueous solution until no bubbles emerge, separate the organic layer, wash once with 50mL 20% sodium carbonate solution, wash twice with 50mL water, dry the organic layer with anhydrous sodium sulfate, filter, and evaporate the solvent under reduced pressure to obtain oily salicylic acid Methyl Esters Derivatives II.
[0027] (2) Preparation of Intermediate III
[0028] After dissolving methyl salicylate derivative II with 300mL acetone, add 40g (0.289mol) of anhydrous potassium carbonate, add 50mL epichlorohydrin under stirring, heat up, reflux for 30 hours, cool to room temperature, filter, evaporate Remov...
Embodiment 2
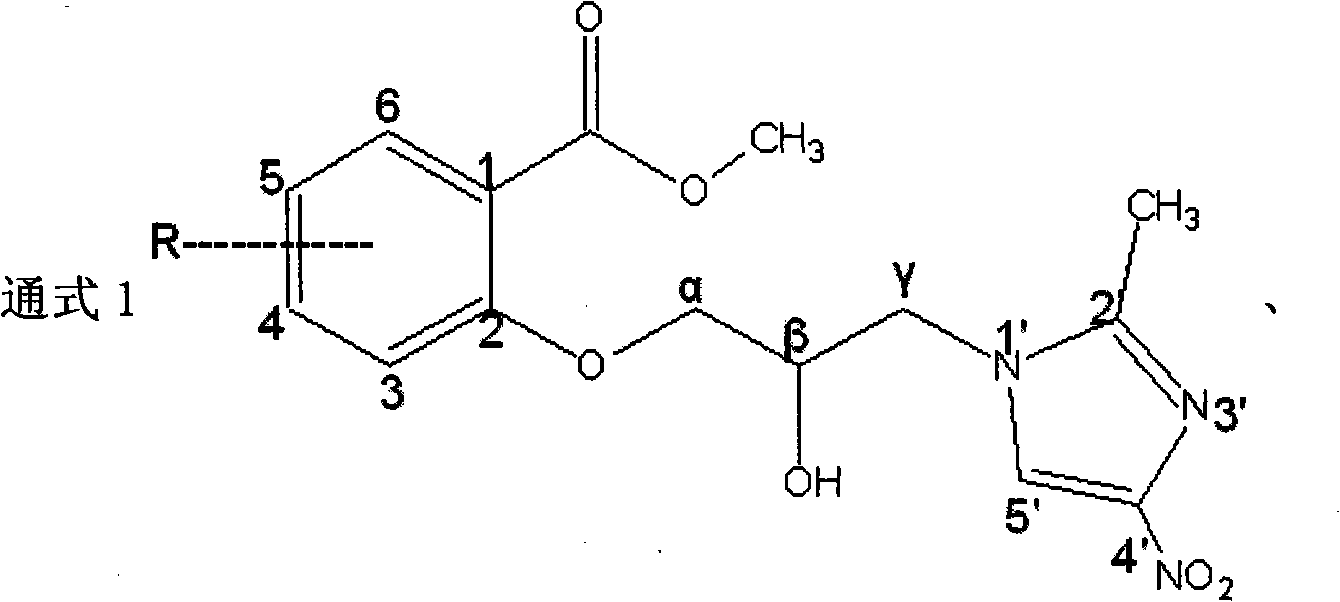
[0029] The secnidazole derivative of embodiment 2 general formula 1 and preparation method thereof
[0030] The secnidazole derivatives of the general formula 1 are 4-nitroimidazole analogues, which belong to esters and are called a series compounds. (1) Preparation of Compound 1a
[0031] Referring to Example 1, using salicylic acid as the starting material to prepare the corresponding methyl salicylate derivatives II and intermediate III; weigh 10g (0.079mol) 2-methyl-5-nitroimidazole, dissolve in Add 18g (0.130mol) of anhydrous potassium carbonate to 70mL of DMF, slowly add (0.158mol) intermediate III dropwise, after the addition is complete, heat up to 90°C, react for 7 hours, evaporate most of the DMF, add ethyl acetate to dissolve, wash with water Three times, the ethyl acetate layer was separated and dried overnight with anhydrous sodium sulfate, and the solvent was evaporated to obtain a solid, which was recrystallized with absolute ethanol to obtain 18.5 g of a white...
Embodiment 3
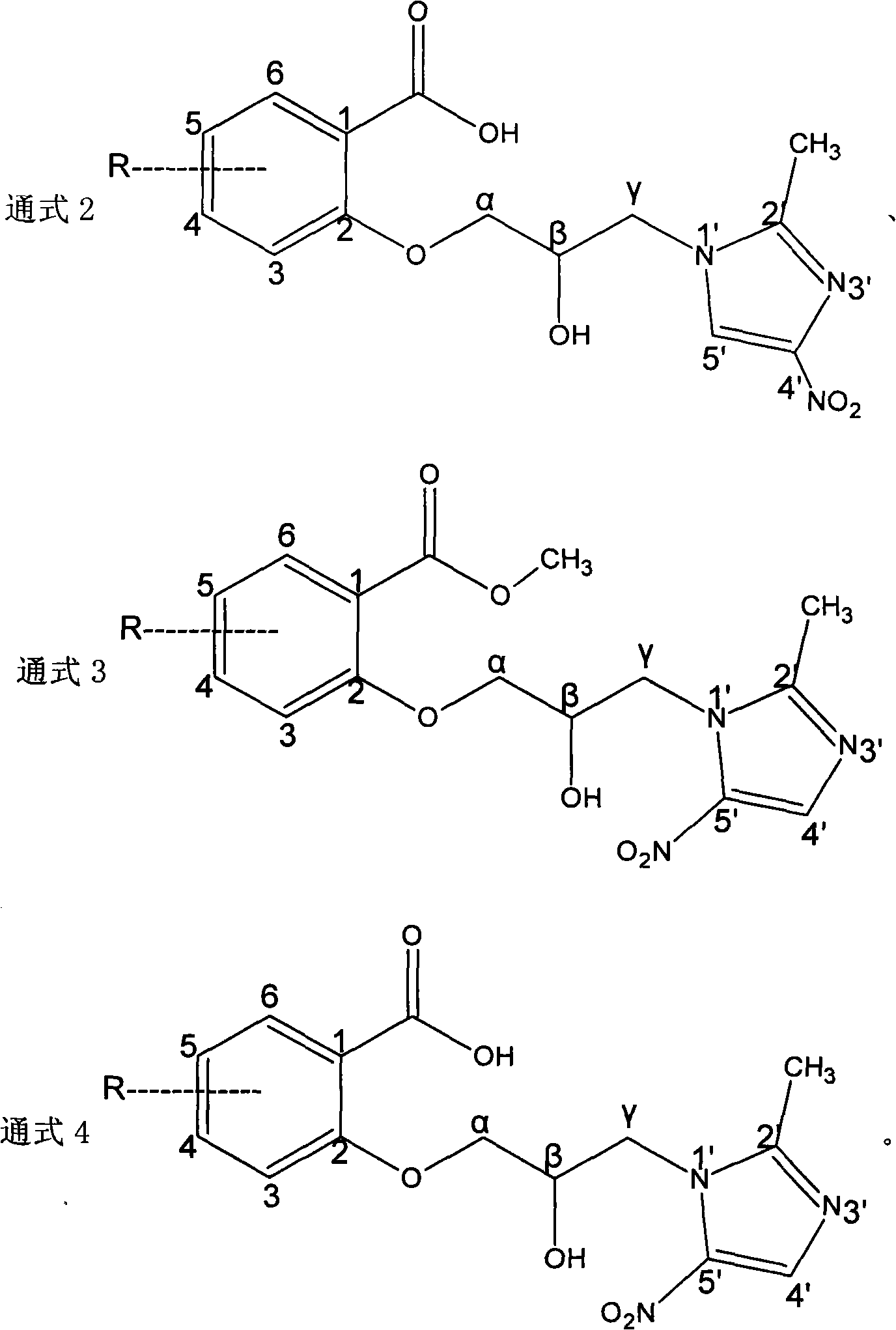
[0042] The secnidazole derivative of embodiment 3 general formula 2 and preparation method thereof
[0043] The secnidazole derivatives of the general formula 2 are hydrolyzed from a series of compounds, which belong to acids and are called b series compounds.
[0044] (1) Preparation of compound 1b
[0045] Take (0.005mol) compound 1a and dissolve it in 25mL ethanol, add 25mL 2mol / L NaOH solution, stir, react at room temperature for 5 hours, adjust the pH to 3 with 10% dilute hydrochloric acid solution, solid precipitates, filter, and crystallize with absolute ethanol to obtain 1.1 g of yellow solid, yield 65%. 1b: MS (ESI) m / Z: 322 (M+1) + , 1 H NMR (DMSO, 600MHz): δ2.39(s, 3H, -2'-CH 3 ), 3.93-4.11 (m, 2H, -NCH 2 -), 4.13-4.16 (m, 2H, -OCH 2 -), 4.33(dd, J=17.8, 6.8Hz, 1H, -CH(OH)-), 5.63(s, 1H, -OH), 7.04(t, J=7.7Hz, 1H, ArH-5), 7.17(d, J=8.2Hz, 1H, ArH-3), 7.51-7.54(m, J=8.2, 1.8Hz, 1H, ArH-4), 7.72(dd, J=7.7, 1.8Hz, 1H, ArH -6), 8.22 (s, 1H, imidazole CH-5'); 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com