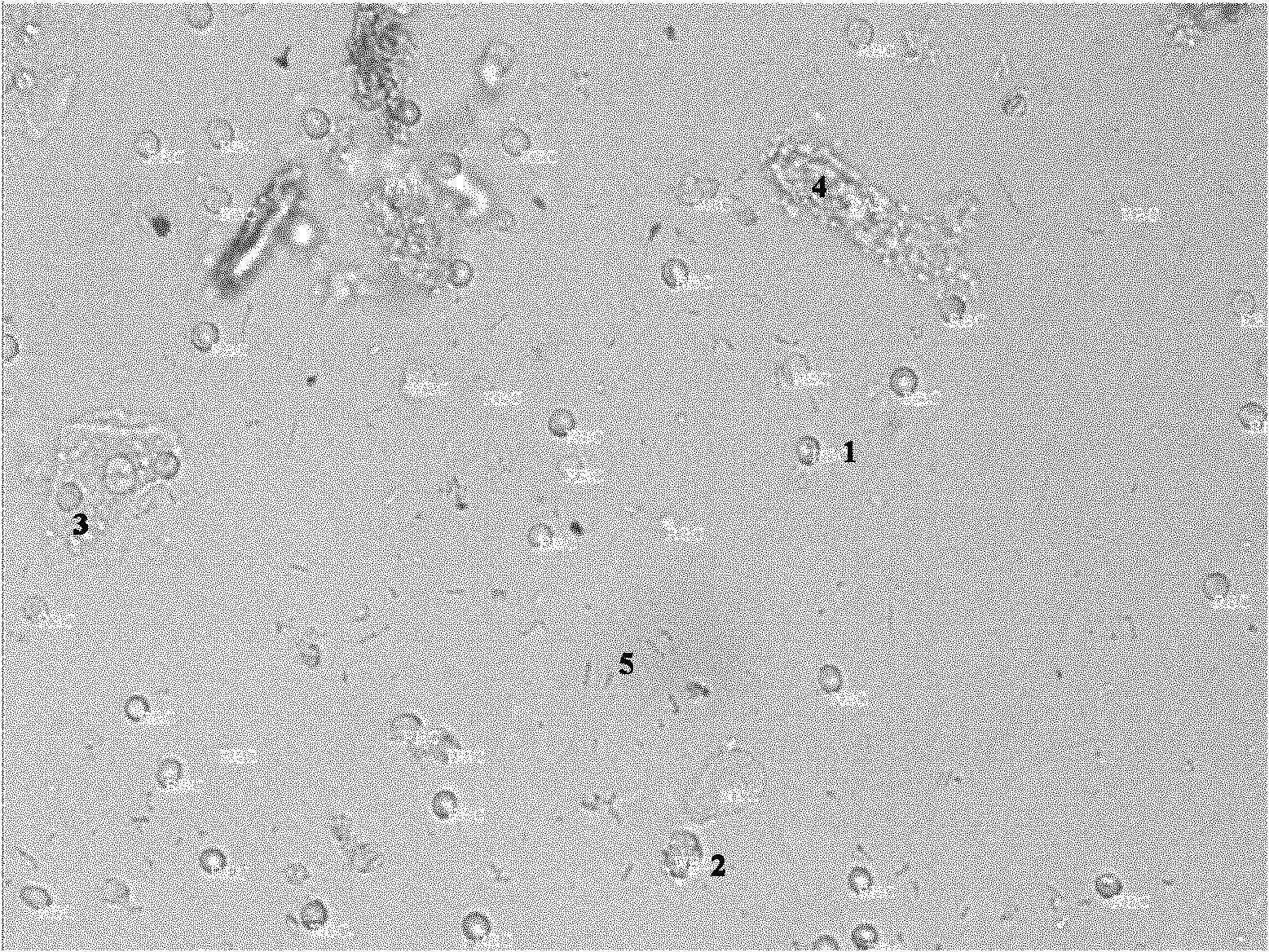Visible urine component quality control product composition and preparation method thereof
A composition and control product technology, applied in the field of urine formed component quality control product composition and its preparation, can solve the problems of few components and can not be used for morphological observation, etc., to achieve easy identification, maintain natural attributes, and good stability period Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
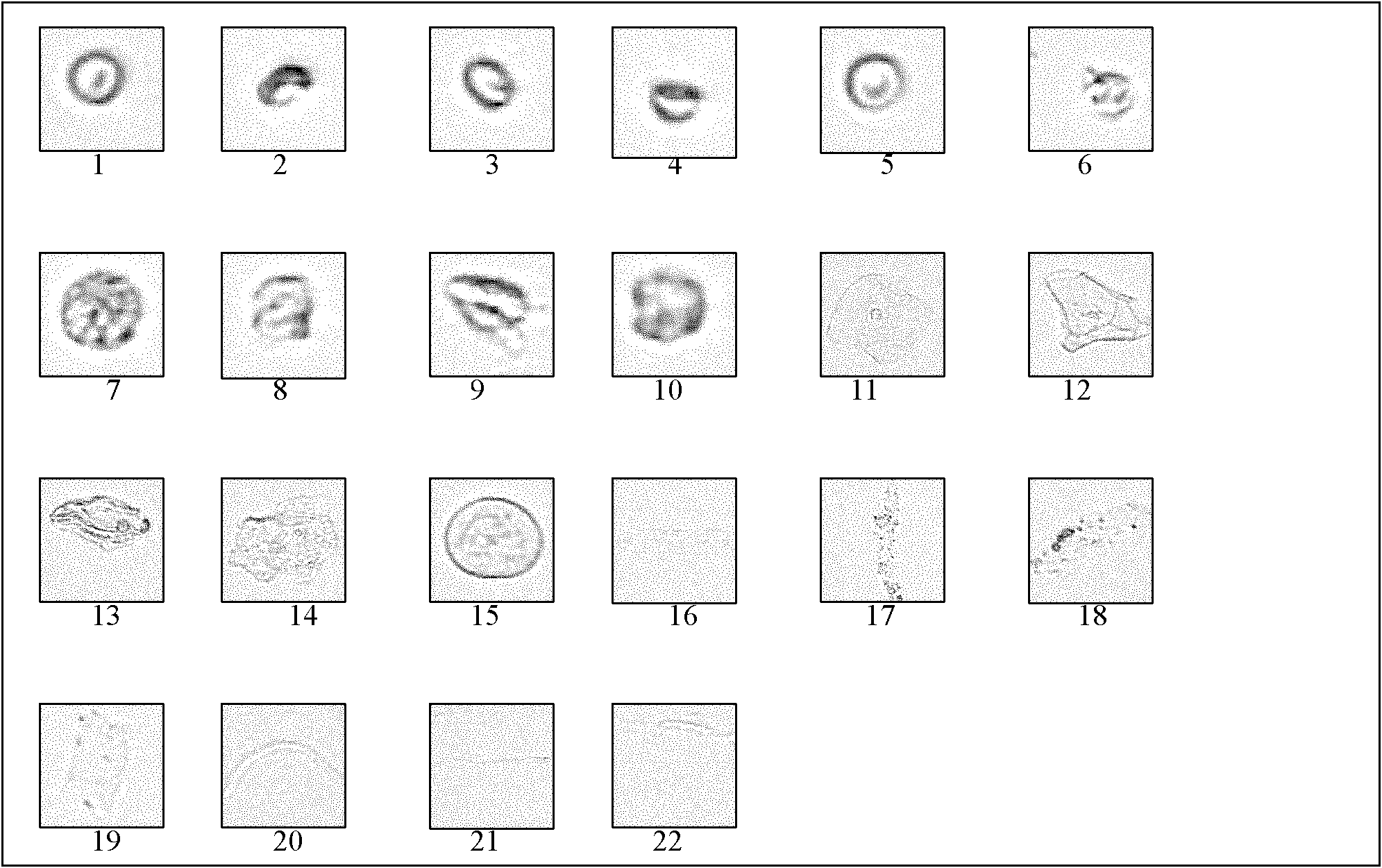
[0030] Preparation of Quality Control Products of Urine Formed Components
[0031] 1. Choose a plastic bottle with a volume of about 2 liters, a cap, and no particles, and sterilize it by high-pressure steam for use.
[0032] 2. Preparation of 10% normal saline formaldehyde solution
[0033] Formaldehyde 100mL plus normal saline 900mL and mix well.
[0034] 3. Preparation of PH7.0 Phosphate Buffer
[0035] Na 2 HPO 4 2H 2 O 108.61g+NaH 2 PO4·2H 2 O 60.86g was dissolved, and the volume was adjusted to 1L.
[0036] 4. Preparation of PH7.0 neutral formaldehyde solution
[0037] Add 20g of formaldehyde to the above 3 and mix well.
[0038] 5. Add 10% normal saline formaldehyde solution to urine at a ratio of 1:20 into an autoclaved plastic bottle, and keep urine for 12 hours.
[0039] 6. Collect urine and transfer it according to biosafety requirements.
[0040] 7. After fully mixing the urine, pour it into the prepared Buchner funnel with filter membrane for suction fi...
Embodiment 2
[0046] Repeatability Monitoring of Quality Control Products of Urine Formed Components
[0047] 1. Properly dilute the urine formed component quality control substance prepared in Example 1 with neutral phosphate buffered saline.
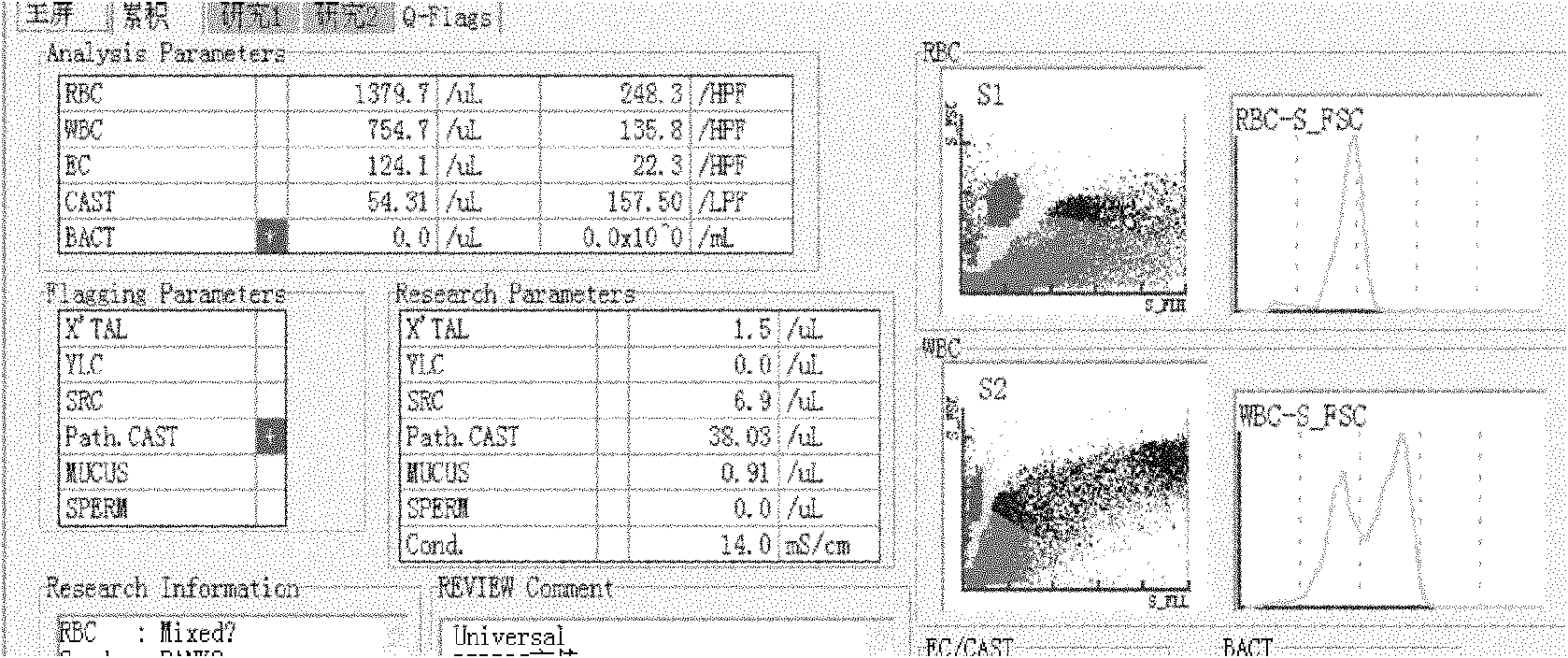
[0048] 2. The low concentration of red blood cells is 10-20 cells / μL, the medium concentration is 20-50 cells / μL, and the high concentration is >100 cells / μL;
[0049] The low concentration of white blood cells is 10-20 / μL, the medium concentration is 20-50 / μL, and the high concentration is >100 / μL;
[0050] The low concentration of epithelial cells is 20 cells / μL;
[0051] The low concentration of epithelial cells is 20 cells / μL;
[0052] The low concentration of the cast is 2-3 / μL, the medium concentration is 3-5 / μL, and the high concentration is >5 / μL.
[0053] 3. The measurement was repeated 12 times on the Sysmex UF500 Urine Formed Components Analyzer in Japan, and the respective mean, standard deviation (s) and imprecision (cv%) were calculat...
Embodiment 3
[0058] Urine Formed Component Quality Control Stability Monitoring
[0059] 1. After fully mixing the urine formed component quality control product prepared according to Example 1, divide it into 2 mL tubes.
[0060] 2. Monitor the aliquoted urine formed component quality control product once a week on the Japan Sysmex UF500 urine formed component analyzer. A total of 8 weeks were measured.
[0061] 3. For the time-dependent changes of different parameters of the urine organic quality control products we made, see the regression fitting curve analysis results, see Table 2.
[0062] The results showed no significant change in urine formed components over 8 weeks.
[0063] Table 2 Analysis results of SPSS13.0 regression fitting curve of different parameter monitoring values
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com