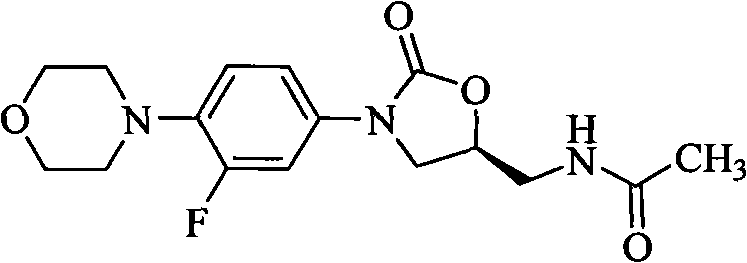
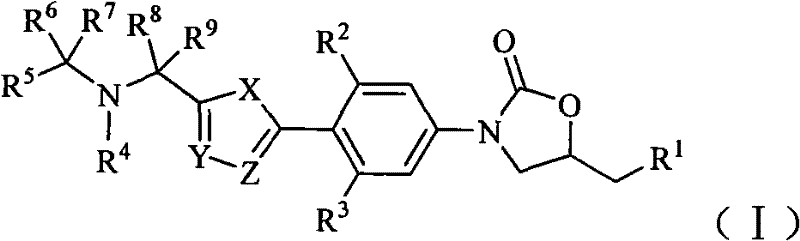
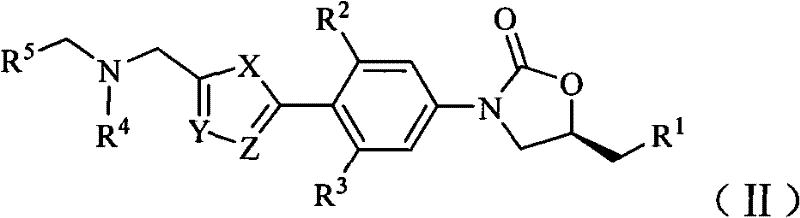
Oxazolidinone bacteriophage containing azaheterocycle
A technology of isoxazolidoxy and alkyl groups, applied in the field of medicine, can solve the problems of severe, single variety of oxazolidinone antibiotics, and continuous emergence of linezolid resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1 (S)-N-[[3-[4-[5-[[(1H-1,2,3-triazol-5-yl)methylamino]methyl]thiophen-2-yl] -3-Fluorophenyl]-2- Preparation of Carbonyloxazolidin-5-yl]methyl]acetamide Hydrochloride (Compound 1 Hydrochloride)
[0122]
[0123] (1) Preparation of N-[(5-bromothiophen-2-yl)methyl]propyn-2-yl-1-amine
[0124]
[0125] In a dry reaction flask, add propargylamine 9.5g (172mmol), 5-bromothiophene-2-carbaldehyde 30g (158mmol) and 1,2-dichloroethane 450mL, stir at room temperature for 2h, then add triethoxy 43.3 g (203 mmol) of sodium borohydride was stirred overnight at room temperature, the reaction solution was washed with water, and the organic phase was directly used in the next step without further treatment.
[0126] (2) Preparation of tert-butyl (5-bromothiophen-2-yl) methyl (propyn-2-yl) carbamate
[0127]
[0128] Add 14.5 g (143 mmol) of triethylamine to the organic solution obtained in the previous step, cool to 0°C, and dropwise add (Boc) 2 O acid anhydride ...
Embodiment 2
[0146] Example 2 (5R)-3-[4-[5-[[(1H-1,2,3-triazol-5-yl)methylamino]methyl]thiophen-2-yl]-3- Fluorobenzene Preparation of -5-(hydroxymethyl)oxazolidin-2-one (compound 4)
[0147]
[0148] (1) tert-butyl[5-[2-fluoro-4-[(R)-5-(hydroxymethyl)-2-oxo-3-oxazolidinyl]phenyl]thiophen-2-yl] Preparation of carbamate
[0149]
[0150] (R)-3-[3-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-5-( Hydroxymethyl) oxazolidin-2-one 8g (23.7mmol), tert-butyl (5-bromothien-2-yl) carbamate 6.9g (23.7mmol), sodium carbonate 5g (47.5mmol), Pd (dppf)Cl 2 Dissolve 0.8g in dioxane, heat to 90°C under the protection of nitrogen and react overnight, cool the reaction solution to room temperature, add water and extract with ethyl acetate, wash the organic layer three times with saline, combine the organic layers, concentrate and pass through the column layer Analysis and separation (DCM:MeOH=20:1) gave 6g of the product.
[0151] (2) Preparation of (R)-3-[4-[5-(aminomethyl)th...
Embodiment 3
[0162] Example 3 (5R)-5-[(1H-1,2,3-triazol-1-yl)methyl]-3-[4-[5-[[(1H-1,2,3- Triazol-5-yl)methylamino] Preparation of methyl]thiophen-2-yl]-3-fluorophenyl]oxazolidin-2-one (compound 5)
[0163]
[0164] (1) (R)-[3-(4-bromo-3-fluorobenzene)-2-carbonyl-oxazolidin-5-yl]methyl 4-methylbenzenesulfonate
[0165]
[0166] Dissolve (R)-3-(4-bromo-3-fluorobenzene)-5-(hydroxymethyl)oxazolidin-2-one (20 g, 69.2 mol) in DCM, add triethylamine (21 g, 207.6mmol) and TosCl (15.8g, 83.1mmol), the reaction solution was stirred overnight at room temperature. The reaction solution was washed with saturated sodium bicarbonate, the organic layer was concentrated to obtain a solid, washed three times with dichloromethane, filtered and dried to obtain a white solid (R)-[3-(4-bromo-3-fluorobenzene)-2-carbonyl-oxane oxazolidin-5-yl]methyl 4-methylbenzenesulfonate 24g.
[0167] (2) (R)-5-(azidomethyl)-3-(4-bromo-3-fluorophenyl)oxazolidin-2-one
[0168]
[0169] (R)-[3-(4-Bromo-3-fluoro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com