Copolymerization method of ethane/alpha-alkene
A technology of copolymerization and olefins, applied in the field of catalysts, can solve the problems of low product yield and long preparation route, and achieve the effect of short synthesis route, high product yield and high insertion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
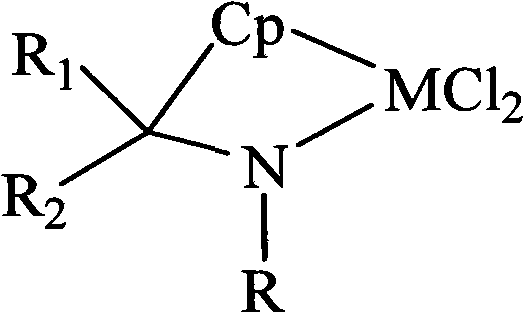
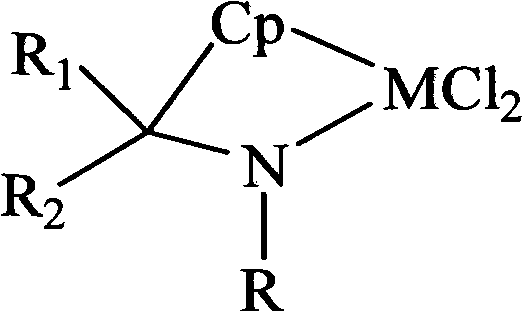
[0037] Contains [t-BuNC(Me) 2 (η 5 -C 5 h 4 )] (ZrCl 2 ) of the mixture of
[0038] Take 20mmol (1.46g) of tert-butylamine, add it to 30mL of THF, stir at -10°C, add 12mL (20mmol) of 1.67M n-BuLi n-hexane solution, and react for 40 hours to obtain a white turbid suspension. After adding 20mmol (2.14g) of 6,6-dimethylfulvene, the solution immediately became clear. After 50 hours of reaction, 12mL (20mmol) of 1.67M n-BuLi in n-hexane was added and reacted for 40 hours to obtain a white turbid suspension. solution, and then 17mmol (3.957g) of ZrCl was added at 0°C 4 , continue to stir and react for 72 hours, the upper layer of the solution becomes clear, the solvent is removed under reduced pressure, and after washing with dichloromethane, it is sucked dry to obtain 5.6 g of a yellow solid with a yield of 84%; 1 H NMR, elemental analysis, and mass spectrometry prove that the product contains [t-BuNC(Me) 2 (η 5 -C 5 h 4 )] (ZrCl 2 ), [t-BuNC(Me) 2 (η 5 -C 5 h 4 )] (...
Embodiment 2
[0040] Contains [t-BuNC(Me) 2 (η 5 -C 5 h 4 )] (TiCl 2 ) of the mixture of
[0041] Take 20mmol (1.46g) of tert-butylamine, add it to 55mL of THF, stir at -10°C, slowly add 12mL of 1.67M n-BuLi n-hexane solution, stir for 30 hours to obtain a white turbid suspension, add 20mmol of 6,6-dimethylfulvene solution immediately became clear, continued to stir and react for 50 hours, then added 12mL of 1.67M n-BuLi n-hexane solution, stirred and reacted for 50 hours to obtain a white suspension, then added 15.5mmol TiCl 4 ·2THF, continue stirring for 50 hours, the solution turns olive green. The solvent was removed under reduced pressure, n-hexane was added to extract excess n-BuLi, and the remaining solid was sucked dry to obtain 5.09 g of olive green solids, with a yield of 80%. through 1 H NMR, elemental analysis, and mass spectrometry prove that the product contains [t-BuNC(Me) 2 (η 5 -C 5 h 4 )] (TiCl 2 ), [t-BuNC(Me) 2 (η 5 -C 5 h 4 )] (TiCl 2 ) molar content i...
Embodiment 3
[0043] Contains [t-BuNC(CH 2 ) 5 (η 5 -C 5 h 4 )] (ZrCl 2 ) of the mixture of
[0044] Take 20mmol (1.46g) of tert-butylamine, add it to 30mL of THF, stir at -10°C, add 12mL (20mmol) of 1.67M n-BuLi n-hexane solution, and stir for 40 hours to obtain a white turbid suspension. Add 20mmol (2.48g) of 6,6-pentamethylenefulvene, the solution becomes clear, stir and react for 48 hours, then add 12mL of 1.67M n-BuLi n-hexane solution, stir and react for 50 hours to obtain a white turbid suspension solution, and then add 18.8mmol of ZrCl 4 , stirred and reacted for 72 hours; after standing still, the white LiCl was removed by filtration, the filtrate was decompressed to remove the solvent, washed with dichloromethane and then sucked dry to obtain 5.5 g of a light yellow solid product. [t-BuNC(CH 2 ) 5 (η 5 -C 5 h 4 )] (ZrCl 2 ) molar content is 84.5%, namely [t-BuNC(CH 2 ) 5 (η 5 -C 5 h 4 )] (ZrCl 2 ) to the rest of the mixture in a molar ratio of 5.45:1; the meltin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com