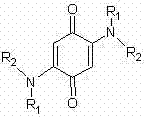
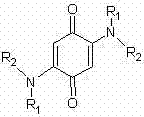
Synthesis method of 2,5-bis(substituted amino)-1,4-benzoquinone compound
A disubstituted, amino technology, applied in the field of synthetic organic compounds, can solve the problems of long reaction time and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of 2,5-dimethylamino-1,4-benzoquinone (1).
[0029] Take 0.5g (0.00454mol) of hydroquinone and add 10ml of ethanol to dissolve it. Take 30% methylamine in ethanol solution, of which methylamine is 1.88g (0.0182mol), add 10ml of ethanol to dilute (reaction molar ratio is hydroquinone:methylamine=1:4), dilute the diluted methylamine alcohol solution Slowly drop the hydroquinone solution, the color of the mixture turns from light brown to brown. Heated in a water bath, the temperature was controlled at 55°C, and the reaction time was 3h. Track and detect the reaction with TLC, the developer is ethyl acetate: sherwood oil=5:1, R f =0.667. After 30 min of reaction, the solution turned from light red to dark red. After the reaction, a red powdery product was formed in the cooled reaction liquid. Filter to obtain crude product. Recrystallize from ethanol. Air-dried to obtain a red powdery pure product, observed under a microscope as red crystals. T...
Embodiment 2
[0030] Example 2: Synthesis of 2,5-bis(dimethylamino)-1,4-benzoquinone (2).
[0031] Take 0.5g (0.00454mol) of hydroquinone and add 10ml of ethanol to dissolve it. Weigh the aqueous solution of dimethylamine, in which dimethylamine is 2.485g (0.01816mol), add 10ml of ethanol to dilute (reaction molar ratio is hydroquinone: dimethylamine = 1:4). Slowly drop the diluted alcohol solution of dimethylamine solution into the ethanol solution of hydroquinone, and the color of the mixture changes from light brown to brown. Heated in a water bath, the temperature was controlled at 55°C, and the reaction time was 2h. TLC tracking detection reaction, the developer is ethyl acetate: petroleum ether = 5:1, R f = 0.62. After reacting for 25 min, the solution turned from brown to dark red. After the reaction was finished, it was cooled, and no solid was generated in the reaction solution, and the color of the solution was reddish black. The reaction solution was sealed and placed in the...
Embodiment 3
[0032] Example 3: Synthesis of 2,5-diethylamino-1,4-benzoquinone (3).
[0033] Take 0.5g (0.00454mol) of hydroquinone and add 10ml of ethanol to dissolve it. Weigh the ethylamine aqueous solution, in which the ethylamine is 0.584g (0.00908mol), add 10ml of ethanol to dilute (reaction molar ratio is hydroquinone:ethylamine=1:2). Slowly drop the diluted ethylamine solution into the ethanol solution of hydroquinone, and the color of the mixture changes from light brown to brown. Heated in a water bath, the temperature was controlled at 52°C, and the reaction time was 6h. TLC tracking detection, the developer is ethyl acetate:petroleum ether=5:1, R f = 0.67. After 30 min of reaction, the solution turned from light red to dark red. After the reaction was finished, it was cooled, and no solid matter was generated in the reaction liquid. Seal the reaction solution and put it in the refrigerator to cool down. The next day, bright red crystals precipitated and were filtered to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com