Uracil-based compounds, and herbicides comprising same
A compound, uracil technology, applied in the field of novel uracil compounds and their preparation, can solve the problem of herbicide loss and achieve high selective herbicidal effect and excellent herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
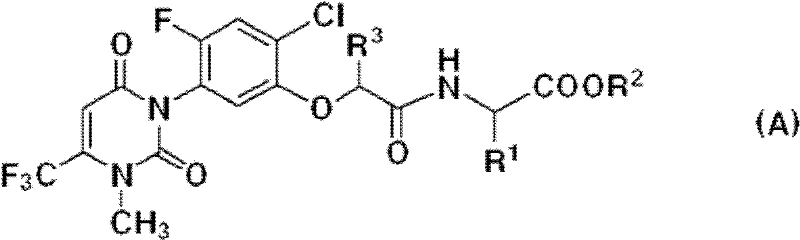
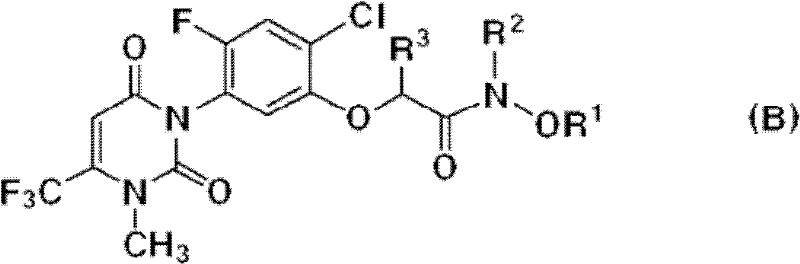
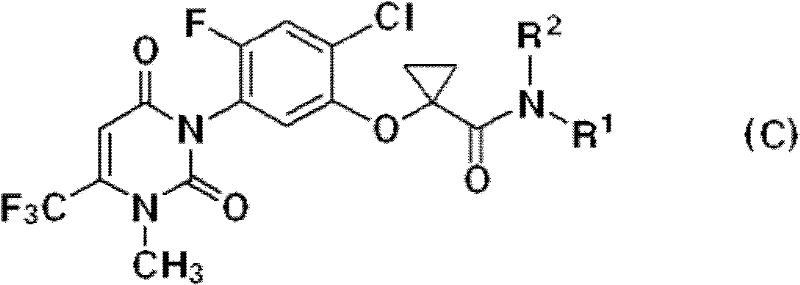
[0029] The uracil compound represented by the chemical formula 1 according to the present invention includes agrochemically acceptable salts, racemates, enantiomers or diastereomeric compounds of the compound represented by the chemical formula 1.
[0030] In addition, the uracil-based compound represented by the chemical formula 1 according to the present invention may be used in the form of an agrochemically acceptable salt. The agrochemically acceptable salts may include, for example, salts with metal salts, organic bases, inorganic acids, organic acids, basic or acidic amino acids, and the like. Suitable metal salts may include, for example, alkali metal salts such as sodium salts, potassium salts and the like; alkaline earth metal salts such as calcium salts, magnesium salts, barium salts and the like; aluminum salts and the like. Salts with organic bases may include, for example, with trimethylamine, triethylamine, pyridine, picoline, 2,6-lutidine, ethanolamine, diethano...
preparation example 1
[0114] [Preparation Example 1: Synthesis of 2-chloropropionyl chloride]
[0115] To a solution obtained by dissolving 10.8 g of 2-chloropropionic acid in 0.5 mL of dimethylformamide, 17.8 g of thionyl chloride (SOCl2) chloride was added dropwise at 50° C. for 30 minutes. After stirring at the same temperature for 7 hours, distillation was carried out at 112° C. to obtain 9.4 g of pale yellow oil.
[0116] 1 H NMR (CDCl 3 , 300MHz) δ 4.68 (q, 1H, J=7.0Hz), 1.84 (d, 3H, J=7.0Hz).
preparation example 2
[0117] [Preparation Example 2: Synthesis of 3-(2-chloropropionylamino)propionic acid methyl ester]
[0118] To a suspension in which 2.25 g of β-alanine methyl ester hydrochloride was suspended in 10 mL of methylene chloride was added 1.63 g of triethylamine, followed by stirring for 30 minutes. After adding 1.27 g of pyridine and cooling the reaction mixture to 0° C., 2.04 g of 2-chloropropionyl chloride was dissolved in 10 mL of methylene chloride and added dropwise for 20 minutes. The reaction mixture was stirred at room temperature for 2 hours, washed successively with water, 1N hydrochloric acid, saturated sodium bicarbonate, dehydrated, filtered, and concentrated to give 2.9 g of a yellow oil in 93% yield.
[0119] 1 H NMR (CDCl3 , 300MHz) δ7.15(br s, 1H), 4.41(q, 1H, J=7.0Hz), 3.74(s, 3H), 3.58(q, 2H, J=6.1Hz), 2.59(t, 2H, J=6.0 Hz), 1.74 (d, 3H, J=7.1 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com