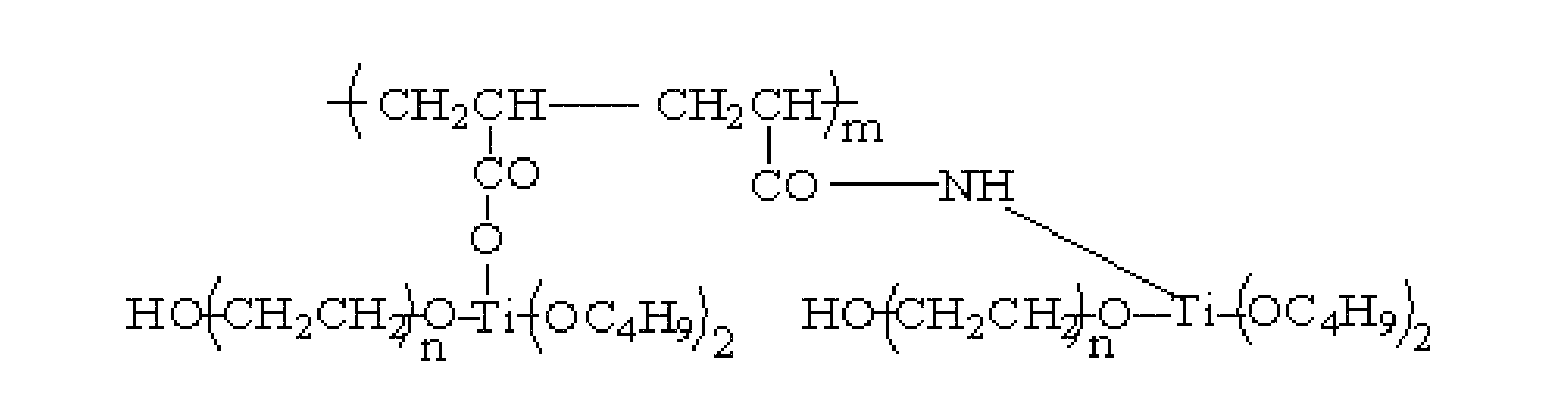
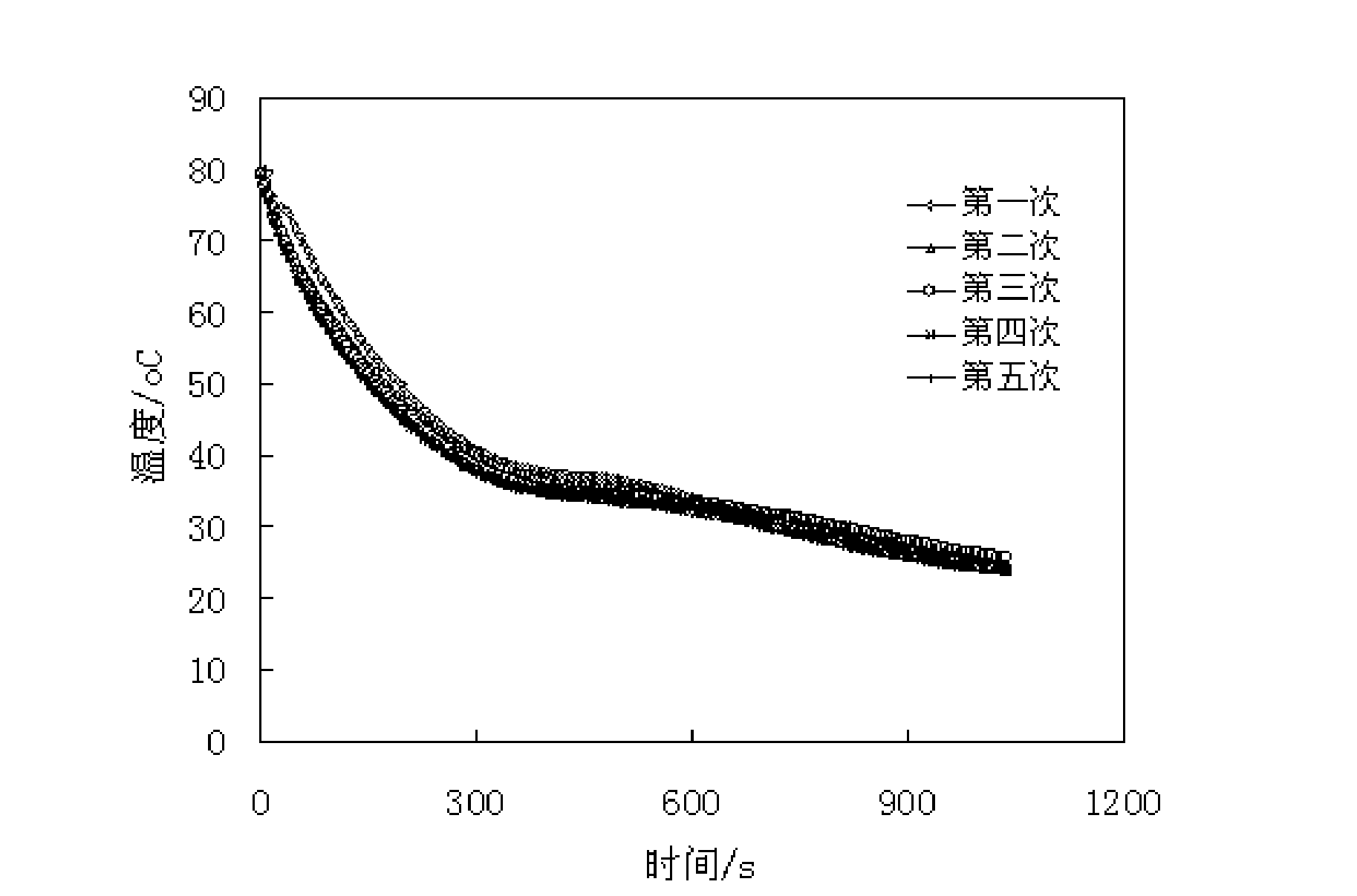
Method for preparing high phase transition enthalpy phase transition temperature-adjusting fiber from hydrolysis products of waste acrylic yarn
A technology of hydrolyzate and acrylic fiber waste, which is applied in the direction of fiber chemical characteristics, chemical instruments and methods, heat exchange materials, etc., can solve the problems of complicated microcapsule preparation process, limited fiber addition, and low heat storage capacity, and achieve Realize the effect of resource reuse, low price and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The hydrolyzed product of acrylic waste silk, that is, hydrolyzed polyacrylonitrile, was placed in DMF to prepare a 10wt.% solution A, which was swollen at 95°C for 180 minutes;
[0023] Dissolve polyethylene glycol (number-average molecular weight 2000, twice the mass of hydrolyzed polyacrylonitrile) in DMF to prepare 20wt.% solution B, dissolve at 70°C for 60min, add butyl titanate and February Dibutyltin silicate, the addition of both is 0.1% of polyethylene glycol quality;
[0024] Mix solution A and solution B evenly, let stand for reaction for 15 minutes, filter under reduced pressure, extract with acetone, and dry in vacuum to obtain graft copolymer polyethylene glycol grafted hydrolyzed polyacrylonitrile (H-PAN-g-PEG) ;
[0025] Dissolve 15 grams of H-PAN-g-PEG in 83 grams of water, add 2 grams of boric acid after all the dissolution, continue to stir to dissolve it completely, and obtain the spinning stock solution after standing for defoaming; the spinning st...
Embodiment 2
[0027] The hydrolyzed product of acrylic fiber waste, that is, hydrolyzed polyacrylonitrile, was placed in DMF to prepare a 15wt.% solution A, which was swollen at 80°C for 180 minutes;
[0028] Dissolve polyethylene glycol (number average molecular weight 2000, twice the mass of hydrolyzed polyacrylonitrile) in DMF to prepare 25wt.% solution B, dissolve at 90°C for 60min, add butyl titanate and February Dibutyltin silicate, the addition of both is 0.1% of polyethylene glycol quality;
[0029] Mix solution A and solution B evenly, let stand for reaction for 10 minutes, filter under reduced pressure, extract with acetone, and dry in vacuum to obtain graft copolymer polyethylene glycol grafted hydrolyzed polyacrylonitrile (H-PAN-g-PEG) ;
[0030] Dissolve 15 grams of H-PAN-g-PEG in 81 grams of water, add 4 grams of boric acid after all the dissolution, continue to stir until it is completely dissolved, and obtain the spinning stock solution after standing for defoaming; pass th...
Embodiment 3
[0032] The hydrolyzed product of acrylic fiber waste, that is, hydrolyzed polyacrylonitrile, was placed in DMF to prepare a 13wt.% solution A, which was swollen at 90°C for 180 minutes;
[0033] Dissolve polyethylene glycol (number-average molecular weight 4000, twice the mass of hydrolyzed polyacrylonitrile) in DMF to prepare 23wt.% solution B, dissolve at 80°C for 60 minutes, add butyl titanate and February Dibutyltin silicate, the addition of both is 0.1% of polyethylene glycol quality;
[0034] Mix solution A and solution B evenly, leave to react for 20 minutes, filter under reduced pressure, extract with acetone, and dry in vacuum to obtain graft copolymer polyethylene glycol grafted hydrolyzed polyacrylonitrile (H-PAN-g-PEG) ;
[0035] Dissolve 20 grams of H-PAN-g-PEG in 78 grams of water, add 2 grams of boric acid after fully dissolved, continue to stir until it is completely dissolved, and obtain the spinning stock solution after standing for defoaming; pass the spinn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com