Application of bisbibenzyl compound to preparation of anti-inflammatory medicament
An anti-inflammatory drug, bisbibenzyl technology, applied in the directions of anti-inflammatory agents, antitumor drugs, drug combinations, etc., can solve problems such as no application of bisbibenzyl compounds, no reports of anti-inflammatory effects of the two compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
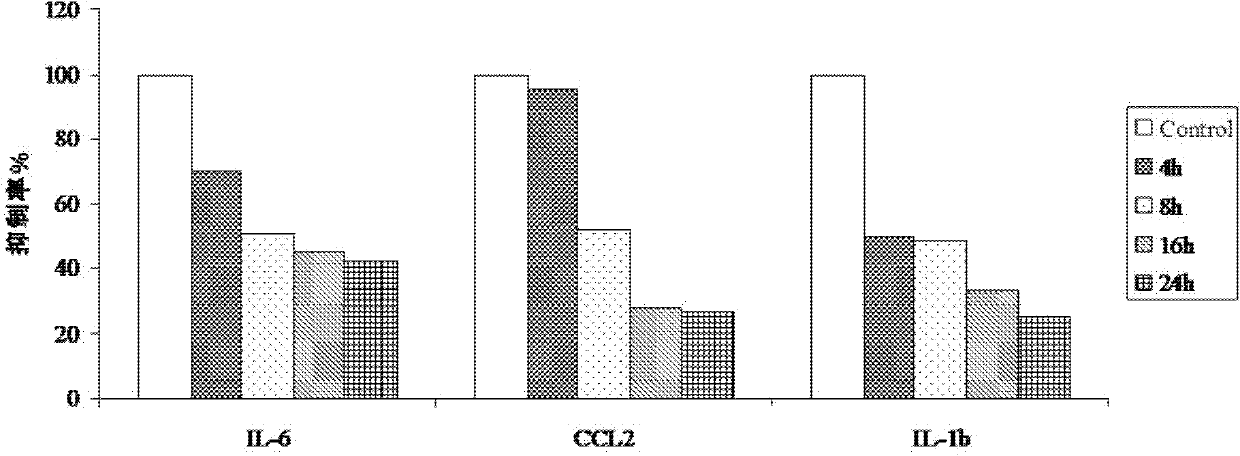
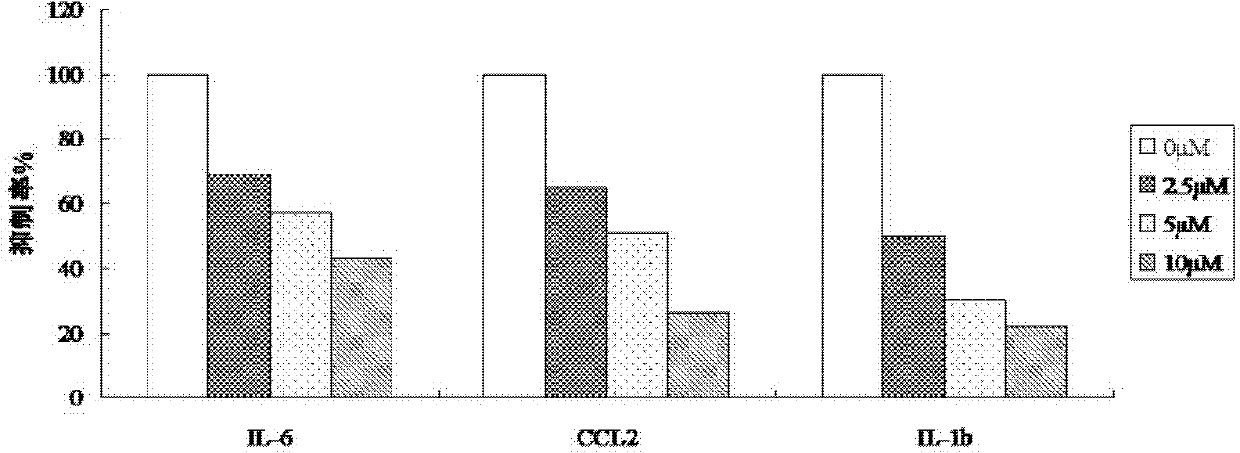
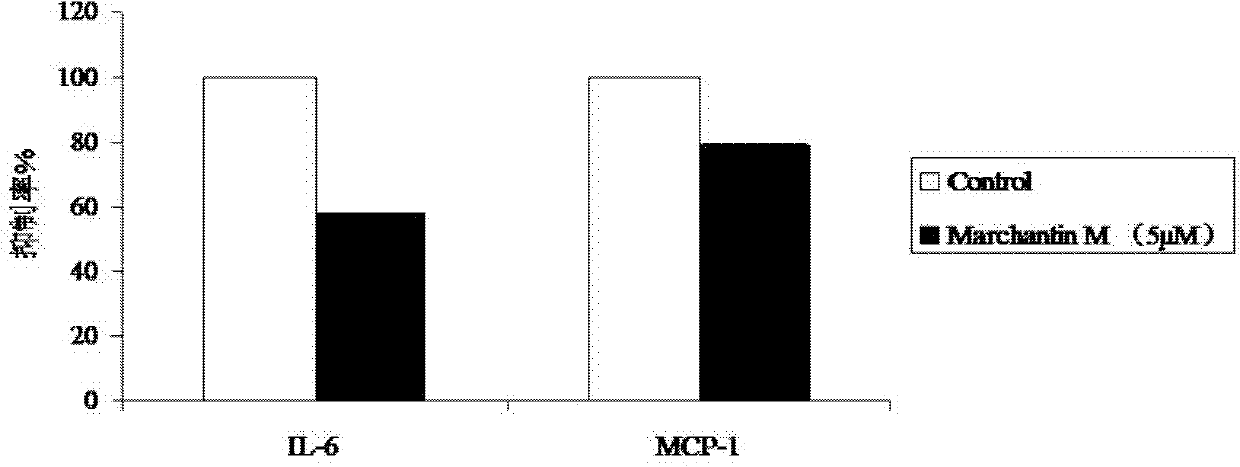
[0031] Example 1: Determination of the levels of IL-6, CCL2, IL-1b and MCP-1 inhibited by dichantin M
[0032] Methods: (1) Isolation and culture of human umbilical vein endothelial cells (HUVEC)
[0033] Take the umbilical cord of the newborn under aseptic conditions, cut off the clamp mark, hematoma, and clot blocking part in the ultra-clean bench, divide it into a 20cm section, use a No. 5 handle disposable intravenous infusion needle to absorb 36 ℃ preheated double-antibody PBS, and insert The stump of the umbilical vein was ligated and fixed at the same time, the residual blood in the vein was rinsed repeatedly, and then the other end of the umbilical cord was clamped with a vascular clamp, and then the prepared 0.25% trypsin and 0.53mM EDTA digestive solution were injected respectively to fill the lumen. 37°C, act for 15 minutes. Loosen one end of the vascular clamp, collect the digestive fluid in the umbilical vein, and then inject EGM2 culture solution containing 5% n...
Embodiment 2
[0038] Example 2: Dichanin M inhibits the gene expression of inflammatory factors such as IL-6, IL-1b, CCL2, COX-2, Icam and TNF
[0039] Methods: (1) RT-PCR detection of mRNA levels
[0040] Total RNA was extracted with Qiagen RNEasy kit, and the total RNA was reverse-transcribed into cDNA (invitrogen Carlsbad, CA) using random primers and Superscript III reverse transcriptase according to the instructions. The reverse transcription reaction conditions were 60 min at 48°C and 15 min at 70°C. TaqMan Universe PCR Master Mix is used for quantitative PCR amplification using pre-synthesized targeting primers. IL-6(Hs_m1), IL-1b(Hs_m1), TNF alpha(Hs_m1), CCL2 / MPC1(Hs_m1), vEGF(Hs_m1), COX-2(Hs_m1). cDNA amplification was performed in 96-well reaction plates using the ABI PRISM 7700 Sequence Detection System.
[0041] The reaction system is shown in Table 1 below:
[0042] Table 1
[0043]
[0044] Thermal cycle amplification is performed and cDNA will be generated followi...
Embodiment 3
[0049] Example 3: Dichanin M inhibits the expression of NF-kB
[0050] Methods: Nuclear protein extraction was carried out using a nuclear extraction kit (Active Motif Tokyo, Japan) according to the instructions. Wash cells with ice-bathed PBS containing phosphatase inhibitors, resuspend cells in 200μl lysate, add 1% NP-40 12.5μl after ice-bath for 15min, centrifuge for 10s to mix well, then centrifuge at 4℃ 13,000rpm, 5min. The pellet was resuspended in 25 μl of nuclear extraction buffer, placed in an ice bath for 15 minutes and shaken to fully lyse, centrifuged at 13,000 rpm at 4°C for 5 minutes, and the supernatant was collected for 5 minutes and stored at -70°C. Bradford method was used to detect nucleoprotein content.
[0051] Treat HUVEC cells overnight with compounds and LPS as described above, extract nuclear protein and measure protein concentration by Bradford method, take 5ug and add an equal volume of sample buffer to boil together for 5min, load the sample, sepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com