L-glutamine organogel containing 1-cyanotoluylene and preparation method thereof
A cyanostilbene, glutamine technology, applied in the field of supramolecular chemistry, can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
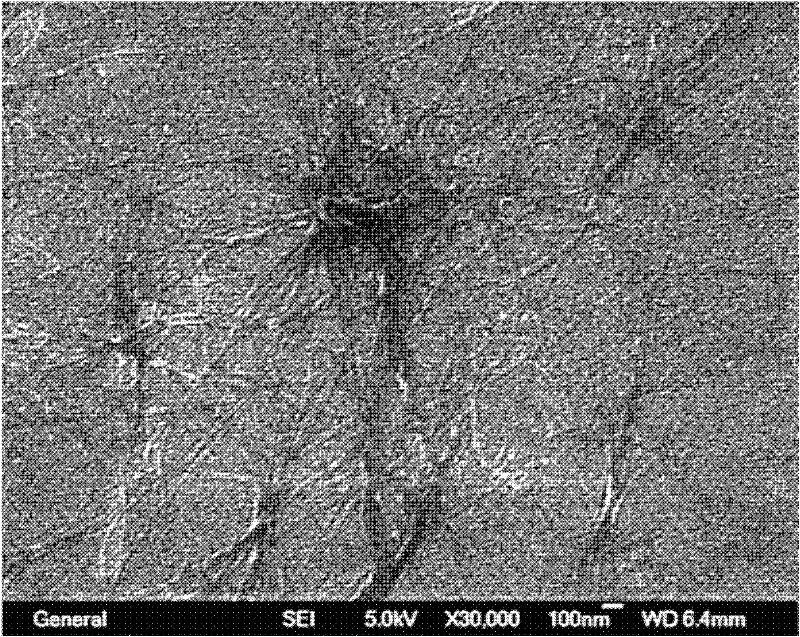
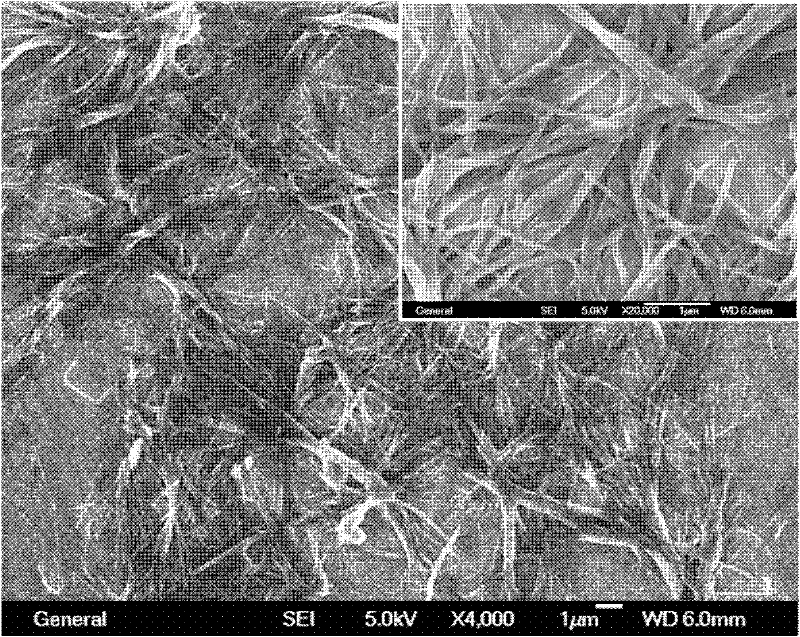
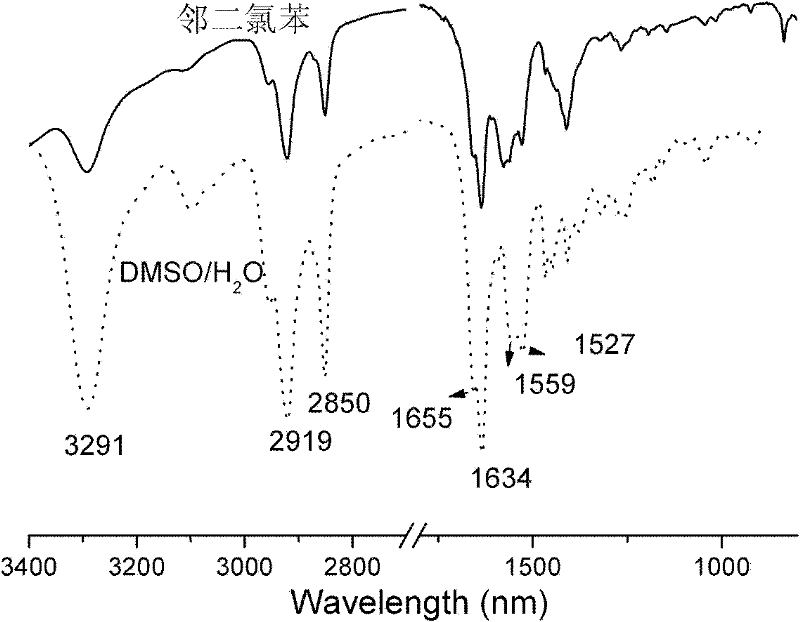
Image
Examples
Embodiment 1
[0029]
[0030] N 1 -((1,3-Di(dodecylcarbamoyl)propylcarbamoyl))-N 5 - Preparation of (4-(1-cyano-2-styryl)phenyl)glutaramide (1):
[0031] Compound 2 (1g, 1.4mmol, synthesized according to literature, "Xue PC, Lu R, Yang XC, et al., Chem. Eur. J., 2009, 15, 9824; Xue PC, Lu R, Zhao L, et al. al., Langmuir, 2010, 26, 6669 ".") and benzaldehyde (0.2g, 1.9mmol) were placed in 50mL of ethanol, heated to reflux at 80°C for 30min, and then added 20 microliters of tetrabutylammonium hydroxide Ethanol solution (concentration is 2mol / L), reflux overnight. Suction filtration, the solid was washed several times with water and ethanol, and then recrystallized twice with ethanol. Vacuum dried to obtain 1.0 g light yellow solid, namely N 1 -((1,3-Di(dodecylcarbamoyl)propylcarbamoyl))-N 5 -(4-(1-cyano-2-styryl)phenyl)glutaramide. Yield: 89%, melting point >200°C.
[0032] H NMR spectrum (500MHz, CDCl 3, TMS): δ=9.14(s, 1H, NH), 7.87(d, J=9.0Hz, 2H), 7.73(d, J=10.5Hz, 2H), 7.61(d,...
Embodiment 2
[0034] Accurately weigh 1 mg of N prepared in Example 1 1 -((1,3-Di(dodecylcarbamoyl)propylcarbamoyl))-N 5 -(4-(1-cyano-2-styryl)phenyl)glutaramide, placed in a glass bottle with a diameter of 1cm and a volume of 10mL with a screw cap, take 1mL of o-dichlorobenzene with a pipette Put it into the bottle, close the screw cap, heat to 120 degrees Celsius to dissolve all the solids, then cool to room temperature naturally, and then stand for 10 minutes to get N with a concentration of 1 mg / mL. 1 -((1,3-Di(dodecylcarbamoyl)propylcarbamoyl))-N 5 - Clear gel system composed of (4-(1-cyano-2-styryl)phenyl)glutaramide and o-dichlorobenzene.
Embodiment 3
[0036] Accurately weigh 1 mg of N prepared in Example 1 1 -((1,3-Di(dodecylcarbamoyl)propylcarbamoyl))-N 5 -(4-(1-cyano-2-styryl)phenyl)glutaramide, placed in a glass bottle with a diameter of 1cm and a volume of 10mL with a screw cap, pipette 0.9mL of dimethyl Put sulfoxide into the bottle, heat to 150 degrees Celsius to dissolve all the solids, add 0.1mL of water to the above solution with a pipette, cover the screw cap, heat to 100 degrees Celsius to redissolve the solids, and then cool naturally to room temperature , and leave it for another 10 minutes to get N with a concentration of 1 mg / m L 1 -((1,3-Di(dodecylcarbamoyl)propylcarbamoyl))-N 5 -(4-(1-cyano-2-styryl)phenyl)glutaramide and DMSO / H 2 A translucent gel system composed of O (volume ratio = 9:1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com