1-heterocycle-5-substituted phenyl-1-pentanone compound and preparation method and application thereof
A compound, phenyl technology, applied in the field of organic compound synthesis, can solve problems such as unclear active ingredients, and achieve the effects of low cost, good biological control activity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
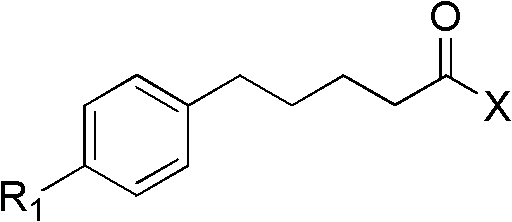
[0030] The compound provided in this example can be prepared according to the following method: 5-substituted phenyl-pentanoic acid and the compound shown by X are subjected to dehydration condensation reaction in an organic solvent in the presence of dicyclohexylcarbodiimide (DCC) , to obtain 1-heterocycle-5-substituted phenyl-1-pentanone compounds, the reaction equation is shown in formula a:
[0031] Reaction equation a:
[0032]
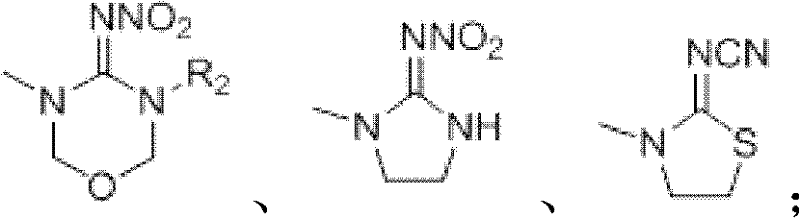
[0033] In the method described in formula a, the source of the compound shown in X as structural formula is:
[0034] 1) When X is 2-cyanoimino-1,3-thiazolidine, it can be purchased through Binhai Xinxing Chemical Co., Ltd.;
[0035] 2) When X is 2-nitroimino-1,3-imidazolidine, it can be purchased through Jiangsu Rudong Shilian Fine Chemical Factory;
[0036] 3) When X is 3-substituent-4-nitroimino-1,3,5-oxadiazine, it can be obtained by reference (USPat.4849432).
[0037] One of the compounds 1-(5-methyl-4-nitroimino-1,3,5-oxadiazin-3-yl)-...
Embodiment 2
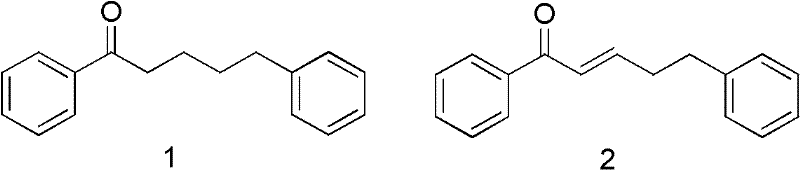
[0051] The positive control agent 1,5-diphenyl-1-pentanone (1), (E)-1,5-diphenyl-2-ene-1-pentanone (2) and the compound sample obtained in Example 1 Weigh 12mg of the compound sample in a 20ml weighing bottle with a ten-thousandth balance, and then use a 1-5ml pipette gun to take 2ml of acetone / methanol (1:1) mixed solvent and add it to the weighing bottle. After it is fully dissolved, add 18ml of an aqueous solution containing 0.1% Tween-80 was mixed thoroughly to obtain a 600 μg / mL assay solution. Using the dipping method. Cut the faba bean plants (leaves of bean seedlings unfolded, plant height about 12 cm) with about 20 bean aphid nymphs that have grown to adults in the same order two days ago from potted plants, and put them in a certain concentration of medicinal solution that has been cultivated in advance. Soak in the medium for 3 seconds, take out and shake off the remaining liquid, insert it on a 12-hole foam plastic plate to moisturize (the foam plastic plate is 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com