Preparation method of seawater electrolytic reaction anode IrO2-RuO2-SnO2-TiO2 nanoparticle coating
An iro2-ruo2-sno2-tio2, nanoparticle technology, applied in electrodes, coatings, electrolysis processes, etc., can solve the problems of expensive active components, uneven distribution of components, and uncertain degree of oxidation, and achieve excellent electrical properties. The effect of catalytic activity and stability, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
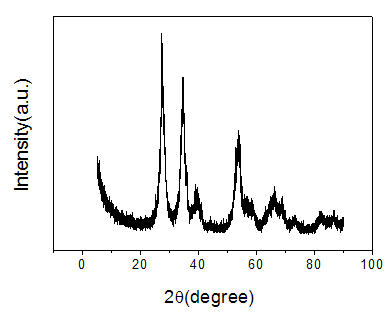
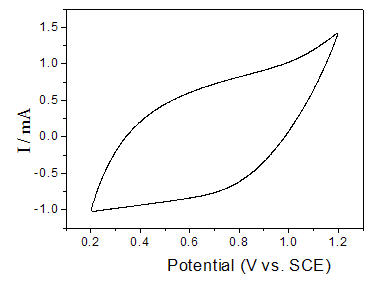
[0032] IrO in Seawater Electrolysis 2 -RuO 2 -SnO 2 -TiO 2 The anode nanoparticle coating is prepared by an improved thermal decomposition method, comprising the following steps:
[0033] 1. Weigh 30mg of titanium dioxide nanoparticles, add 0.18g of SnCl 4 ·5H 2 O, 8ml 0.067mol / L RuCl 3 2H 2 O, 5ml 0.067mol / L H 2 IrCl 4 ·6H 2 O solution, then add 0.001 g / ml of polyacrylamide with a molecular weight of 3,000,000, stir or ultrasonically oscillate to make it evenly mixed to form a suspension to prepare the anode electrode coating solution;
[0034] 2. Brush the suspension prepared in step 1) on the Ti sheet, dry at 120°C, calcinate at 500°C, and cool;
[0035] 3. Repeat step 2) for about 20 times until the weight increase of the Ti sheet reaches 1.2mg / cm 2 , that is, to prepare the anode IrO 2 -RuO 2 -SnO 2 -TiO 2 Nanoparticle coating.
Embodiment 2
[0037] IrO in Seawater Electrolysis 2 -RuO 2 -SnO 2 -TiO 2 The anode nanoparticle coating is prepared by an improved thermal decomposition method, comprising the following steps:
[0038] 1. Weigh 30mg of titanium dioxide nanotubes, add 0.15g of SnCl 4 ·5H 2 O, 8ml0.067mol / L RuCl 3 2H 2 O, 6ml 0.067mol / L H 2 IrCl 4 ·6H 2 O solution, then add polyacrylamide 0.0015g / ml with a molecular weight of 3000000, stir or ultrasonically oscillate to make it evenly mixed to form a suspension, which is to prepare the anode electrode coating solution;
[0039] 2. Brush the suspension prepared in step 1) on the Ti sheet, dry at 120°C, calcinate at 500°C, and cool;
[0040] 3. Repeat step 2) for about 20 times until the weight increase of the Ti sheet reaches 1.4 mg / cm 2 , that is, to prepare the anode IrO 2 -RuO 2 -SnO 2 -TiO 2 Nanoparticle coating.
Embodiment 3
[0042] IrO in Seawater Electrolysis 2 -RuO 2 -SnO 2 -TiO 2 The anode nanoparticle coating is prepared by an improved thermal decomposition method, comprising the following steps:
[0043] 1. Weigh 30mg of titanium dioxide nanofibers, add 0.18g of SnCl 4 ·5H 2 O, 8ml 0.067mol / L RuCl 3 2H 2 O, 5ml 0.067mol / L H 2 IrCl 4 ·6H 2 O solution, then add 0.002 g / ml polyacrylamide with a molecular weight of 10000000, stir or ultrasonically oscillate to make it evenly mixed to form a suspension, which is to prepare the anode electrode coating solution;
[0044] 2. Brush the suspension prepared in step 1) on the Ti sheet, dry at 120°C, calcinate at 600°C, and cool;
[0045] 3. Repeat step 2) for about 20 times until the weight increase of the Ti sheet reaches 1.5mg / cm 2 , that is, to prepare the anode IrO 2 -RuO 2 -SnO 2 -TiO 2 Nanoparticle coating.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com