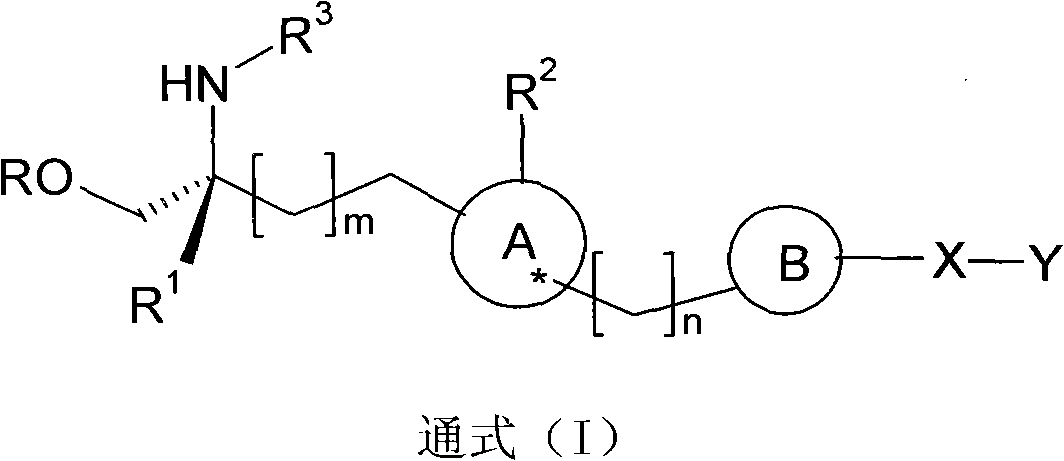
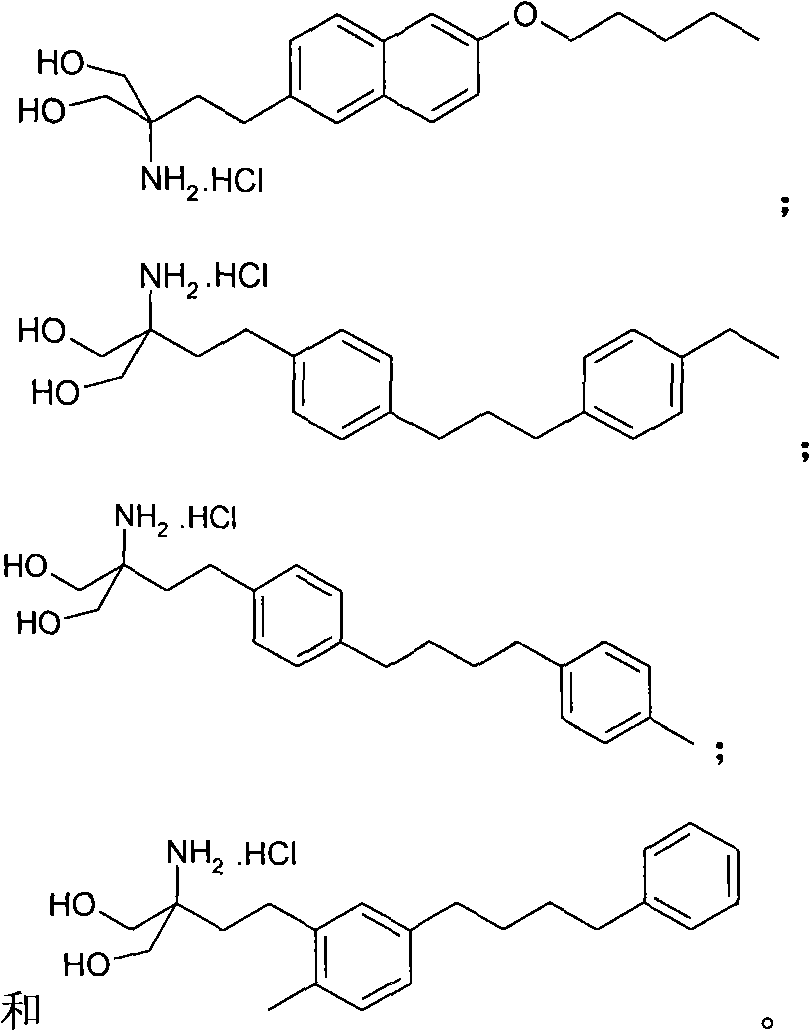
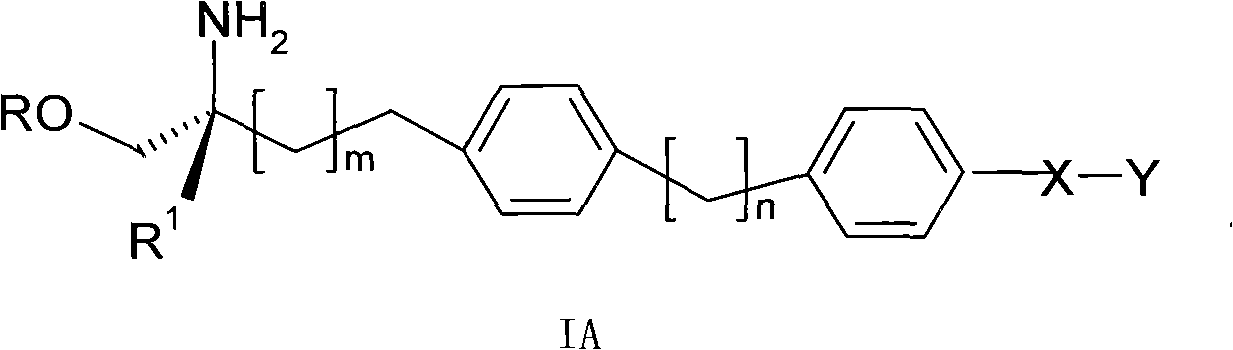
Propylene glycol derivatives, preparation method thereof, pharmaceutical composition and use thereof
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0249] This example demonstrates Preparation of 2-acetamido-2-[2-(4-(7-oxazole-10-phenyl)phenyl)-2-oxo-ethyl]-1,3-malonic acid diethyl ester
[0250] (9-1) Preparation of phenacyl n-phenylbutyrate
[0251]
[0252] Dissolve 4-phenylbutyric acid (12.2g, 74mmol) in 40mL of acetonitrile, add chloroacetylbenzene (5g, 32.5mmol) and triethylamine (6.9g, 68.2mmol), heat to reflux for 4h, evaporate the solvent under reduced pressure , the residue with CH 2 Cl 2 Extracted, washed with water to neutral, anhydrous NaSO 4 After drying, filtering and concentrating, 9 g of yellow oil was obtained, and the product was directly sent to the next step without separation and purification.
[0253] (9-2) Preparation of 2-n-phenylpropyl-4-phenyloxazole
[0254]
[0255] The raw material phenacyl n-phenylbutyrate (9.2 g, 33 mmol) was dissolved in 90 mL of xylene, acetamide (9.7 g, 165 mmol) and 47% of BF 3 Diethyl ether solution 3.2mL, heated to reflux for 40h, evaporated the solvent u...
preparation example 2
[0269] This example demonstrates
[0270] Preparation of 2-amino-2-[2-(4-(6-oxazole-9-phenyl)phenyl)-2-oxo-ethyl]-1,3-propanediol hydrochloride.
[0271] (10-1) Preparation of phenacyl n-phenylpropionate
[0272]
[0273] Dissolve 3-phenylpropionic acid (11.1g, 74mmol) in 40mL of acetonitrile, add chloroacetylbenzene (5g, 32.5mmol) and triethylamine (6.9g, 68.2mmol), heat to reflux for 4h, evaporate the solvent under reduced pressure , the residue with CH 2 Cl 2 Extracted, washed with water to neutral, anhydrous NaSO 4 Dry, filter, and concentrate to obtain 8.5 g of a yellow oil, which is directly sent to the next step without separation and purification.
[0274] 1 HNMR (MERCURY300MHz, CDCl 3 ) δ7.91 (dd, J=8.7Hz, 1.5Hz, 2H, 2ArH) 7.61 (t, J=7.2Hz, 1H, ArH) 7.48 (t, J=7.5Hz, 2H, 2ArH) 7.33-7.18 (m , 5H, 5ArH) 5.34 (s, 2H, CH 2 )3.04(t, J=7.5Hz, 2H, CH 2 ) 2.82 (t, J = 7.5Hz, 2H, CH 2 )
[0275] ESI (m / z) 291 (M+Na + )
[0276] (10-2) Preparation of 2-pheneth...
preparation example 3
[0292] This example demonstrates
[0293] Preparation of 2-amino-2-[2-(4-(4-(4-n-butanol acetate)phenethyl)phenyl)-2-oxo-ethyl]-1,3-propanediol hydrochloride .
[0294] (11-1) Preparation of 4-bibenzyl-4-oxo-butyric acid
[0295]
[0296] Under ice-bath cooling (0 °C), the starting material diphenylethane (19.7 g, 108.8 mmol) was dissolved in 500 mL of dry CH 2 Cl 2 , added ground succinic anhydride (10.8 g, 108.8 mmol), and then slowly added AlCl in portions 3 (31.9g, 240.5mmol), when AlCl 3 After adding all the mixture, continue to stir for 1 hour, point the board to find that the raw material point disappears, add 16mL of concentrated hydrochloric acid and 50mL of water to decompose, evaporate the solvent to dryness, a white solid precipitates, filter with suction, wash the filter cake with 32mL (1:3) diluted hydrochloric acid, Then wash with 4 mL of water. Add the filter cake to the eggplant-shaped bottle, add 13gNa 2 CO 3 Boil with 81mL water for 30min, cool,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com