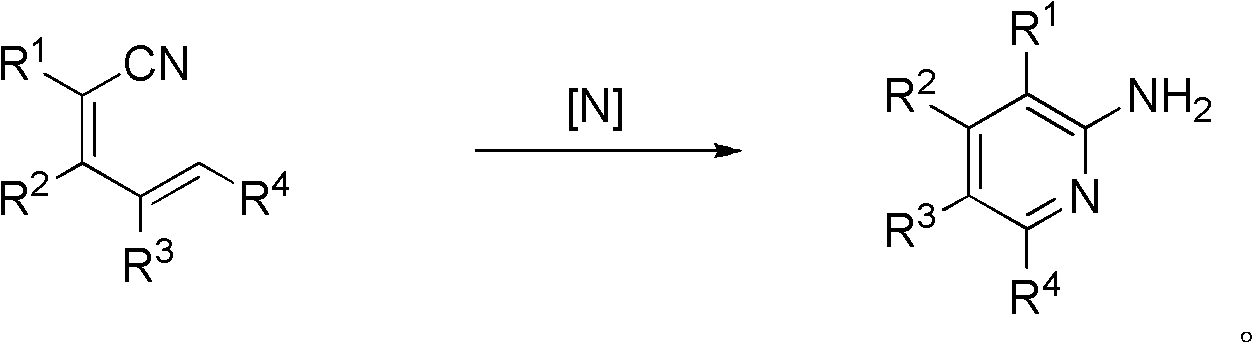
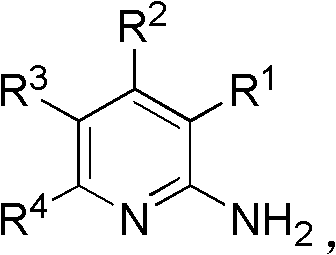
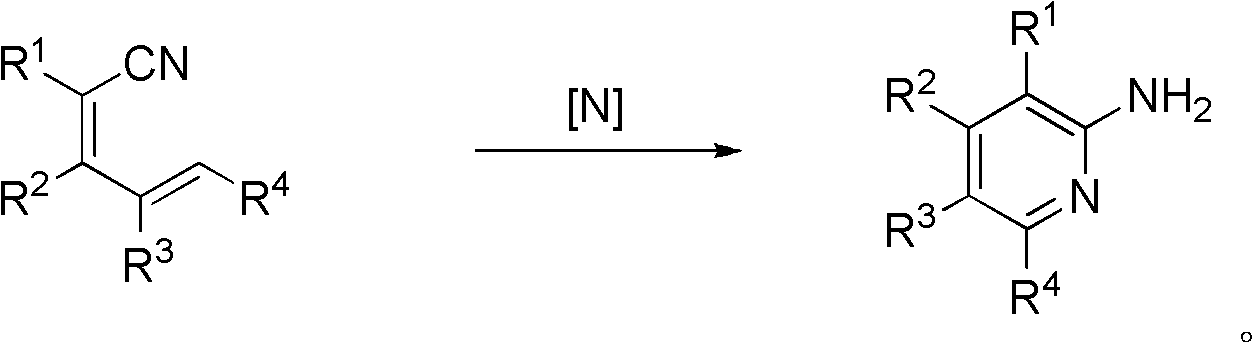
A kind of synthetic method of 2-aminopyridine compounds
A synthesis method, aminopyridine technology, applied in the direction of organic chemistry, etc., can solve the problems of atomic diseconomy, complicated reaction steps, specific substitution position, etc., achieve the effect of light side reaction, simplified synthesis process, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In a 50 ml round bottom flask, add 2 mmol 2-cyano-5-(4-methoxyphenyl)-2,4-pentadienenitrile, 2.1 mmol hydroxylamine hydrochloride, 10 ml dimethyl formaldehyde Amide; Stir at room temperature, add 4.4 mmol triethylamine at the same time, then stir the reaction system at 80°C for 3.5h, the reaction ends; after column chromatography, the white solid product 2-amino-3-cyano- 0.280 g of 6-(4-methoxyphenyl)pyridine, yield 62.2%. The reaction formula is as follows:
[0037]
[0038] Data characterization of the product:
[0039] Mp: 191~192℃;
[0040] 1 H NMR (400MHz, CDCl 3 , 25°C) δ=3.87(3H), 5.19(2H), 6.98(2H), .10(1H), 7.70, (1H), 7.94(2H);
[0041] 13 C NMR (125MHz, CDCl 3 , 25°C) δ=55.4, 88.4, 109.5, 114.2, 117.2, 128.8, 130.3, 141.7, 159.0, 160.0, 161.5.
[0042] It shows that the product is 2-amino-3-cyano-6-(4-methoxyphenyl)pyridine.
Embodiment 2
[0044] In a 50 milliliter round bottom flask, add 2 millimoles of 2-cyano-5-(4-chlorophenyl)-2,4-pentadienenitrile, 2.1 millimoles of hydroxylamine hydrochloride, and 10 milliliters of dimethylformamide; Stir at room temperature, add 4.4 mmol triethylamine at the same time, then stir the reaction system at 80°C for 3.5h, and the reaction ends; after column chromatography, the white solid product 2-amino-3-cyano-6- (4-Chlorophenyl)pyridine 0.290 g, yield 63.1%. The reaction formula is as follows:
[0045]
Embodiment 3
[0047] In a 50 ml round bottom flask, add 2 mmol 2-cyano-3-(3-methoxyphenyl)-2,4-pentadienenitrile, 2.1 mmol hydroxylamine hydrochloride, 10 ml dimethylformaldehyde Amide; Stir at room temperature, add 4.4 mmol triethylamine at the same time, then stir the reaction system at 80°C for 3.5h, and the reaction ends; the white solid product 2-amino-3-cyano-4-(3- 0.301 g of methoxyphenyl) pyridine, yield 66.8%. The reaction formula is as follows:
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com