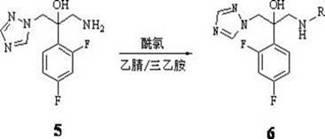
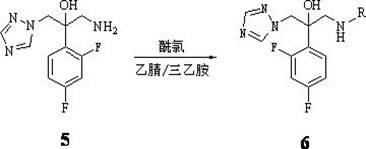
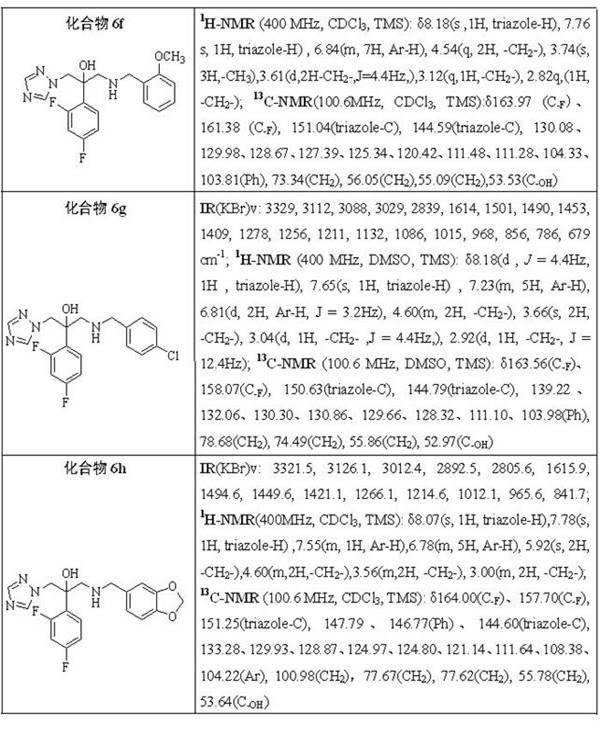
Triazole alcohol compound, its preparation method and application
A compound, triazole technology, applied in organic chemistry, antifungal agents, etc., to achieve novel and effective methods, good antifungal activity in vitro, and the effect of overcoming the problem of fungal drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1), Preparation of 2-chloro-2',4'-difluoroacetophenone (compound 2)
[0045] Add 6.65 g (0.05 mol) of anhydrous aluminum trichloride and 9.9 ml (0.1 mol) of m-difluorobenzene into a 50 mL three-necked flask with a drying tube, then slowly add 7.7 ml (0.1 mol) of chloroacetyl chloride dropwise, at room temperature The reaction was stirred for 4 h. After the reaction was completed, a small amount of water was added under an ice bath, stirred to release heat, and then placed in a refrigerator to cool and crystallize, filtered with suction, and dried to obtain 18.6 g of a white solid (yield 98%), mp 46-48°C.
[0046] (2), prepare α-(1 H -1,2,4-Triazol-1-yl)-2,4-difluoroacetophenone (Compound 3)
[0047] Add 6.36 g (0.06 mol) of anhydrous Na to a 100 mL three-neck flask 2 CO 3 , 8.28 g ( 0.12 mol) of 1 H -1,2,4-triazole and 50 mL of dichloromethane, stirred at room temperature, then added 11.4 g (0.06 mol) of compound 2 , then slowly dropwise added 1.44 g (0.0024 mol...
Embodiment 2
[0052] Embodiment 2: preparation 1-(1 H -1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-propionyl
[0053] Amino)-2-ol (compound 6a )
[0054] In a 25 mL three-neck flask, add 0.254 g (1.0 mmol) of the prepared compound at 0°C 5 , then add 6 mL of anhydrous acetonitrile and stir to dissolve, then add 0.28ml, (2.0 mmol) triethylamine, slowly add 0.09 mL (1.0 mmol) of propionyl chloride solution in 6 mL of anhydrous acetonitrile dropwise under stirring, after the addition is complete, the mixture Turn to room temperature and stir for 2 h to stop the reaction. First filter to remove the white solid, the filtrate was evaporated to dryness under reduced pressure, the residue was dissolved in acetone, and separated by thin layer chromatography to obtain a light yellow solid 1-(1 H -1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-propionyl)-2-ol 243 mg, yield 78%, melting point 183 -184°C.
Embodiment 3
[0055] Embodiment 3: preparation 1-(1 H -1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-benzoyl
[0056] (amino)-2-ol (compound 6b)
[0057] In a 50 mL three-neck flask, add 0.51 g (2.0 mmol) of the prepared compound at 0°C 5 , then add 12 mL of anhydrous acetonitrile, stir to dissolve, then add 0.56 ml, (4.0 mmol) triethylamine, slowly add 0.24 mL (2.0 mmol) of benzoyl chloride solution in 12 mL of anhydrous acetonitrile dropwise under stirring, the addition is complete, The mixture was stirred at room temperature for 3 h to stop the reaction. First filter to remove the white solid, the filtrate was evaporated to dryness under reduced pressure, the residue was dissolved in acetone, and separated by thin layer chromatography to obtain a light yellow solid 1-(1 H -1,2,4-triazol-1-yl)-2-(2,4-difluorophenyl)-3-(N-propionyl)-2-ol 566 mg, yield 79%, melting point 173 -174°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com