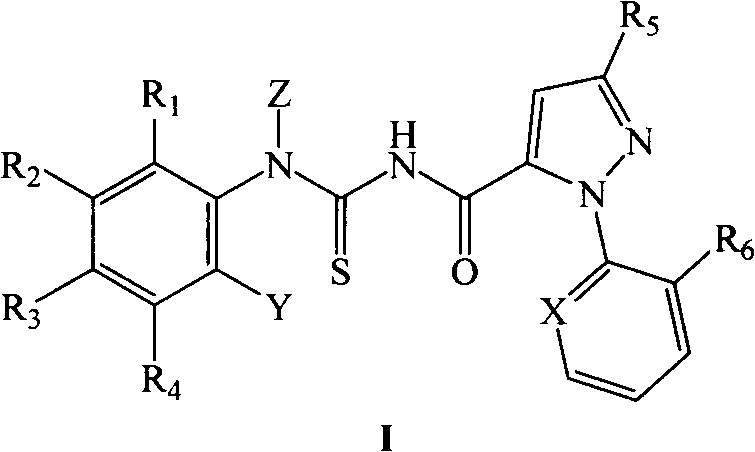
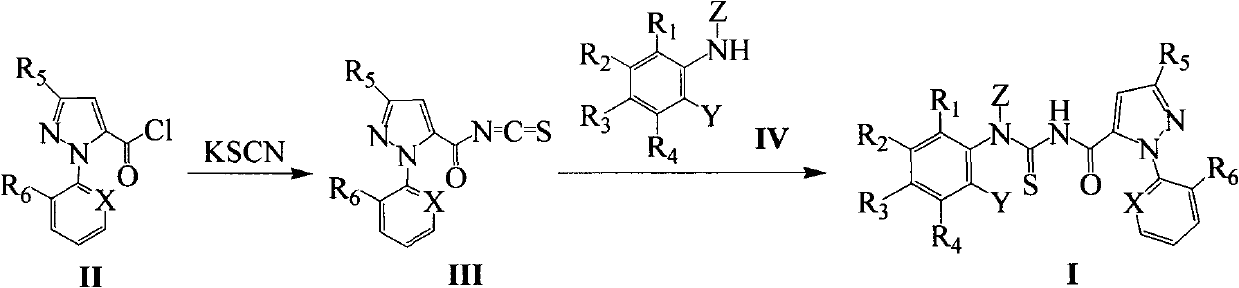
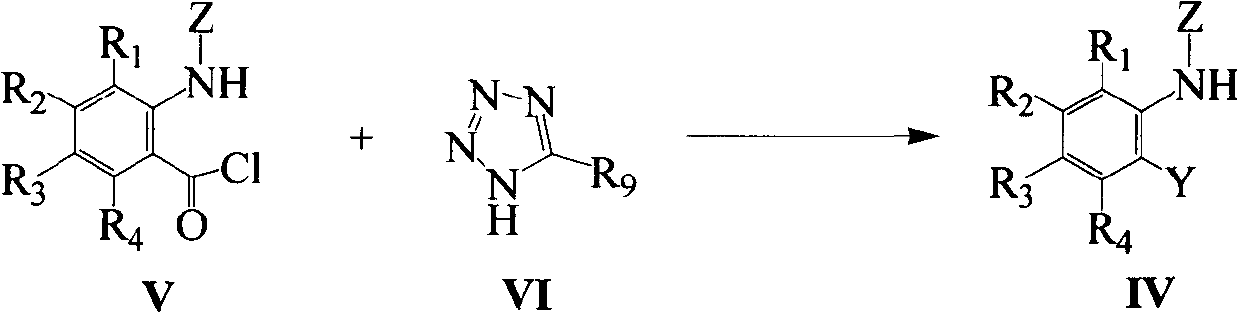
Pyrazole carboxyl thiourea derivative and its preparation method and application
A technology of pyrazole carboxyl thiourea and its derivatives, which is applied in the field of pyrazole carboxyl thiourea derivatives and their preparation, and can solve the problems that the insecticidal activity of pyrazole carboxyl thiourea derivatives has not been disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation method of compound 01.
[0042] Step A: Preparation of 3-chloro-2-hydrazinopyridine
[0043]
[0044] Add 29.6g (0.2mol) of 2,3-dichloropyridine and 120g (1.92mol) of 80% hydrazine hydrate into a 250mL round bottom flask, heat to reflux for 5 hours, cool to room temperature, filter with suction, wash with ethanol, and dry to obtain 27.6g of white crystals, yield 96%, m.p.163-164°C.
[0045] Step B: Preparation of ethyl 1-(3-chloropyridin-2-yl)-3-pyrazolidinone-5-carboxylate
[0046]
[0047] Add 140mL of absolute ethanol to a 250mL three-necked flask, slowly add small pieces of sodium metal (4.83g, 0.21mol), heat to reflux after the reaction is complete, add 27g (0.19mol) of 3-chloro-2-hydrazinopyridine, and then Reflux for 10 minutes, slowly add 36.1 g (0.21 mol) of diethyl maleate dropwise, and then stir and reflux for 30 minutes after the dropping is completed, and after cooling to 65°C, neutralize the reaction mixture with 24 g (0.4 mol) of aceti...
Embodiment 2
[0067] Preparation method of compound 22.
[0068] Step A: Preparation of 1-(furan-2-yl)-4,4,4-trifluoro-1,3-butanedione
[0069]
[0070] Add 20mL of absolute ethanol to a 100mL round bottom flask, slowly add 0.81g (35mmol) of sodium metal, after the reaction is complete, add 3.1g (25mmol) of 2-acetylfuran dissolved in 20mL of absolute ethanol, and stir at room temperature for 1 hour , add 4.2g (30mmol) ethyl trifluoroacetate dropwise under ice bath, then reflux for 24 hours, cool, add 50mL water to dilute, acidify with hydrochloric acid, extract with ethyl acetate (25mL×3), combine the organic phases, and saturated saline After washing, drying with anhydrous sodium sulfate, and precipitation, the residue was subjected to column chromatography to obtain 4.21 g of a red liquid with a yield of 81.7%.
[0071] Step B: Preparation of 1-(3-chloropyridin-2-yl)-3-trifluoromethyl-5-(furan-2-yl)-1H-pyrazole
[0072]
[0073] In a 100mL round bottom flask, add 2.71g (13mmol) 1-(f...
Embodiment 3
[0084] Preparation method of compound 35.
[0085] Step A: Preparation of 3-methyl-2-amino-5-chlorobenzoic acid
[0086]
[0087] Add 10g (66mmol) of 3-methyl-2-aminobenzoic acid and 40mL of DMF into a 100mL three-necked flask, stir to dissolve the solid, then add 8.81g (66mmol) of NCS, heat the reaction solution to 100°C for 40 minutes, cool, Let stand overnight. The reaction solution was slowly poured into 150mL of ice water, the solid was precipitated, filtered with suction, washed with water, then dissolved in 250mL of ethyl acetate, filtered to remove insoluble matter, the filtrate was dried over anhydrous magnesium sulfate, desolvated under reduced pressure, and the residue was used After washing with ether, 8.6 g of a light brown solid was obtained, with a yield of 70.2%, m.p.197°C (decomposition).
[0088] 3-Methyl-2-amino-5-bromobenzoic acid was synthesized in a similar way using NBS as bromination reagent, a yellow solid with a yield of 86%, m.p.213-216°C.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com