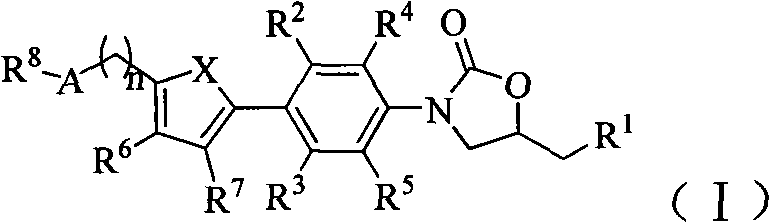
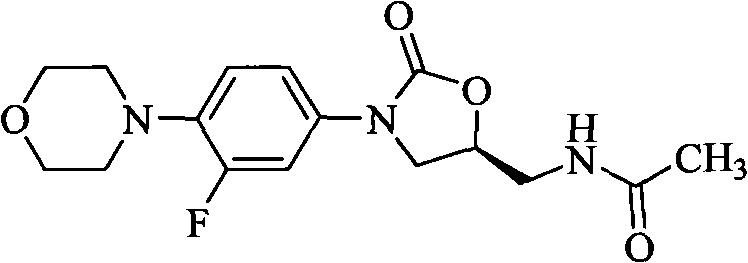
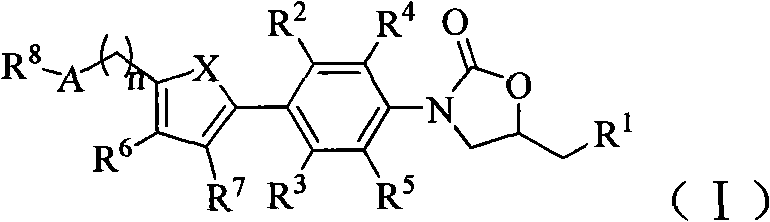
Oxazolidinone antibiotics containing five-membered heterocycles
An alkyl and alkoxy technology, applied in the field of medicine, can solve the problems of the continuous emergence of linezolid resistance, the inability to meet clinical needs, and the single variety of oxazolidinone antibiotics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1N-[[(S)-3-[3-fluoro-4-[5-[(1-methyl-1H-tetrazol-5-yl)methyl]thiophen-2-yl]phenyl] -2-Oxooxazole Preparation of alk-5-yl]methyl]acetamide (compound 1)
[0112]
[0113] (1) Tri-n-butyltin azide
[0114]
[0115] Add 200 mL of tert-butyl methyl ether and tri-n-butyltin chloride (15 g) to NaN 3 (34g) in water (200mL), the mixture was stirred at room temperature for 1 hour, the organic phase was collected by separation and dried over anhydrous magnesium sulfate, and the product was concentrated as an oil (15g, 97%).
[0116] (2) 2-(5-bromothien-2-yl)acetonitrile
[0117]
[0118] At room temperature, NBS (31.6g, 0.178mol) was added to a DMF solution of 2-(thiophen-2-yl)acetonitrile (20g, 0.163mol), and the mixture was refluxed for 5 hours, washed with water and NaOH solution after cooling, The organic phase was dried and concentrated, and the crude product was obtained by column chromatography as an oil (21 g, 64%).
[0119] (3) 5-[(5-bromothiophen...
Embodiment 2
[0148] Example 2N-[[(S)-3-[4-[5-[(1H-tetrazol-5-yl)methyl]thiophen-2-yl]-3-fluorophenyl]-2-oxo Oxazolidin-5-yl] Preparation of methyl] acetamide (compound 2)
[0149]
[0150] (1) 2-(5-bromothien-2-yl)acetonitrile
[0151]
[0152] With reference to Example 1 (2), the product obtained is an oil (21g, 64%).
[0153] (2) 5-[(5-bromothiophen-2-yl)methyl]-1H-tetrazole
[0154]
[0155] With reference to Example 1 (3), the product obtained was a solid (9.06g, 97%).
[0156] (3) N-[[(S)-3-[4-[5-[(1H-tetrazol-5-yl)methyl]thiophen-2-yl]-3-fluorophenyl]-2-oxo Oxazolidin-5-yl]methyl]acetamide
[0157]
[0158] N-[[(S)-3-[3-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl] -2-Oxooxazolidin-5-yl]methyl]acetamide (1.2g, 0.003mol), 5-[(5-bromothiophen-2-yl)methyl]-1H-tetrazole (0.8g , 0.003mol), Na 2 CO 3 (0.68g, 0.006mol) and 5mL water were dissolved in 20mL DMSO, to which 0.5g Pd(dppf) was added 2 Cl 2 , the mixture was reacted at 90° C. under nitroge...
Embodiment 3
[0161] Example 3N-[[(S)-3-[4-[5-[(3H-1,2,3-triazol-4-yl)methyl]thiophen-2-yl]-3-fluorophenyl ]-2-oxooxazolidine Preparation of -5-yl] methyl] acetamide (compound 3)
[0162]
[0163] (1) 2-bromo-5-(chloromethyl)thiophene
[0164]
[0165] Add 30 mL of petroleum ether to concentrated hydrochloric acid (18 mL) and 37% formaldehyde solution (18 mL), cool to 5 ° C, add 2-bromothiophene (30 g, 0.185 mol) to it, and pass HCl gas into it under vigorous stirring And keeping the system temperature below 10°C, the organic phases were combined after petroleum ether extraction and concentrated to obtain a yellow oil (34 g, 87%).
[0166] (2) [3-(5-Bromothiophen-2-yl)-1-propynyl]trimethylsilane
[0167]
[0168] Trimethylsilylacetylene (6.7mL, 0.047mol) and ethylmagnesium bromide (15mL, 0.047mol) were dissolved in THF (30mL), and after the mixture was stirred for 0.5 hours, CuBr (0.3g, 2.4mmol) was added thereto and 2-bromo-5-(chloromethyl)thiophene (10g, 0.047mmol), reflux...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com