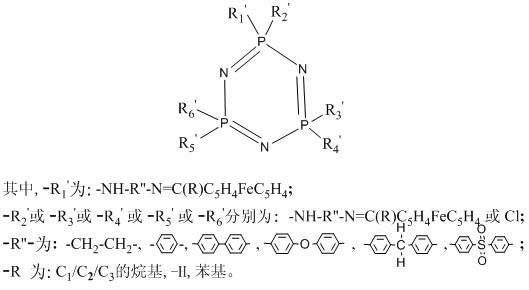
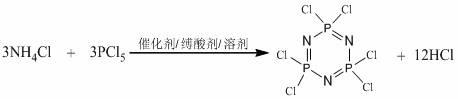
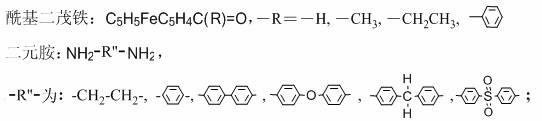Monoacyl ferrocene Schiff base aminocyclotriphosphazene and its synthesis method
A kind of technology of monoacyl ferrocene Schiff base amino cyclotriphosphazene and monoacyl ferrocene Schiff base amino group, which is applied in the field of monoacyl ferrocene Schiff base amino cyclotriphosphazene and its synthesis , can solve the problems of difficult preparation, low reaction temperature, inconvenient operation, etc., and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: The synthesis of monoacyl ferrocene Schiff base aminocyclotriphosphazene is realized through the following steps:
[0031] Synthesis of hexachlorocyclotriphosphazene: In a 500ml four-necked flask equipped with a mechanical stirrer, a condenser, and a thermometer, add finely ground ammonium chloride 30g, 120ml chlorobenzene, 1g zinc oxide, and 20ml pyridine in sequence, and heat and stir To the reflux of chlorobenzene. Then slowly drop into the chlorobenzene solution dissolved with 100g of phosphorus pentachloride for about 2 hours, keep the solution under reflux for 2 to 3 hours until the HCl in the airway tube is obviously weakened and stop heating. Continue stirring to discharge the HCl gas in the system, cool the reaction system, and filter to obtain a light yellow solution. Wash the solution with distilled water for 2 to 3 times until the organic layer is clear and transparent, separate the chlorobenzene layer, add anhydrous sodium sulfate to dry ov...
Embodiment 2
[0037] The synthesis of monoacyl ferrocene Schiff base amino cyclotriphosphazene is realized through the following steps:
[0038] Synthesis of hexachlorocyclotriphosphazene: In a 500ml four-necked flask equipped with a mechanical stirrer, a condenser, and a thermometer, add finely ground 30g ammonium chloride, 120ml chlorobenzene, 1g anhydrous magnesium chloride, and 20ml pyridine in turn, and heat Stir until the chlorobenzene refluxes. Then slowly drop into the chlorobenzene solution dissolved with 100g of phosphorus pentachloride for about 2 hours, keep the solution under reflux for 2 to 3 hours until the HCl in the airway tube is obviously weakened and stop heating. Continue stirring to discharge the HCl gas in the system, cool the reaction system, and filter to obtain a light yellow solution. Wash the solution with distilled water for 2 to 3 times until the organic layer is clear and transparent, separate the chlorobenzene layer, add anhydrous sodium sulfate to dry ove...
Embodiment 3
[0044] The synthesis of monoacyl ferrocene Schiff base amino cyclotriphosphazene is realized through the following steps:
[0045] Synthesis of hexachlorocyclotriphosphazene: In a 500ml four-neck flask equipped with a mechanical stirrer, a condenser, and a thermometer, add finely ground 30g ammonium chloride, 200ml tetrachloroethane, 1g anhydrous zinc chloride, 20ml of pyridine, heated and stirred until tetrachloroethane refluxed. Then add 100g of phosphorus pentachloride in 5 to 10 times, and finish adding in about 2 hours, keep the solution under reflux for 2 to 3 hours, and stop heating when the HCl in the airway tube is obviously weakened. Continue stirring to discharge the HCl gas in the system, cool the reaction system, and filter to obtain a light green solution. Wash the solution with distilled water for 2 to 3 times until the organic layer is clear and transparent, separate the tetrachloroethane layer, add anhydrous sodium sulfate to dry overnight, filter, and dist...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com