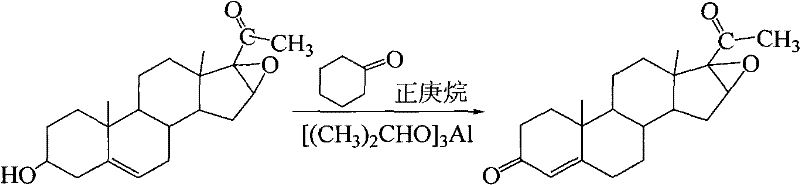
Synthesis of 16α,17α-epoxy-4-pregnene-3,20-dione
A synthetic method, the technology of pregnene, applied in the direction of steroids, organic chemistry, etc., can solve the problems of low yield, high production cost, environmental pollution of toluene vapor, etc., to reduce environmental pollution, reduce production cost, and reduce temperature Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Put 2kg of oxygen bridge into the flask, then add 22L of n-heptane and 8L of cyclohexanone, install a thermometer and a water separator on the flask, heat and stir with water. When there are no drops of water in the water separator, cool down to ≤90°C, add 0.32kg aluminum isopropoxide, cool down to ≤90°C after intense reflux for 4 hours, add dropwise 0.3kgNaOH and 1LH 2 O prepared alkaline water. After the dropwise addition was completed, stir and reflux for 20 minutes, and then remove the lower alkaline water. Then add 0.08kgNaOH and 4.4LH 2 O prepared alkaline water was stirred and refluxed for 30 minutes, and the lower alkaline water was separated. Finally, add 0.044kgNaOH and 4.4LlH 2 The alkaline water prepared by O is distilled at atmospheric pressure to oil-free beads (an appropriate amount of boiling water needs to be added during the distillation). Suction filtration, washing filter cake to neutral. After the filter cake was dried, 1.9 kg of crude product ...
Embodiment 2
[0025] Put 5kg of oxygen bridge into the flask, then add 55L of n-heptane and 20L of cyclohexanone, install a thermometer and a water separator on the flask, heat and stir with water. When there are no drops of water in the water separator, cool down to ≤90°C, add 0.8kg aluminum isopropoxide, cool down to ≤90°C after vigorously refluxing for 4 hours, add dropwise 0.75kgNaOH and 2.5LH 2 O prepared alkaline water. After the dropwise addition was completed, stir and reflux for 20 minutes, and then remove the lower alkaline water. Then add 0.2kgNaOH and 11LH 2 O prepared alkaline water was stirred and refluxed for 30 minutes, and the lower alkaline water was separated. Finally, add 0.11kgNaOH and 11LlH 2 The alkaline water prepared by O is distilled at atmospheric pressure to oil-free beads (an appropriate amount of boiling water needs to be added during the distillation). Suction filtration, washing filter cake to neutral. After the filter cake was dried, 4.76kg of the crude...
Embodiment 3
[0027] Put 8 kg of oxygen bridge into the flask, then add 88 L of n-heptane and 32 L of cyclohexanone, install a thermometer and a water separator on the flask, heat and stir with water. When there are no drops of water in the water separator, cool down to ≤90°C, add 1.28kg of aluminum isopropoxide, cool down to ≤90°C after intense reflux for 4 hours, add dropwise 1.2kgNaOH and 4LH 2 O prepared alkaline water. After the dropwise addition was completed, stir and reflux for 20 minutes, and then remove the lower alkaline water. Then add 0.32kgNaOH and 17.6LH 2 O prepared alkaline water was stirred and refluxed for 30 minutes, and the lower alkaline water was separated. Finally, add 0.176kgNaOH and 17.6LlH 2 The alkaline water prepared by O is distilled at atmospheric pressure to oil-free beads (an appropriate amount of boiling water needs to be added during the distillation). Suction filtration, washing filter cake to neutral. After the filter cake was dried, 7.7 g of crude ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com