Targeted peptide NGR of CD13 (aminopeptidase N) and application thereof
A technology targeting peptides and fusion proteins, applied in the direction of peptides, etc., can solve the problem of not showing anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Design and screen NGR peptides with good binding ability to CD13 by conventional histochemical staining methods:
[0030] (1) Artificial chemical synthesis of the following polypeptides labeled with biotin at the C-terminus (N-terminal → C-terminal sequence):
[0031] CNGRVSTNGRC (SEQ ID NO: 1), CNGRNGRC (SEQ ID NO: 4), CNGRVSTC (SEQ ID NO: 5), CNGRNGRNGRC (SEQ ID NO: 6), CNGRVSTNGRVSTNGRC (SEQ ID NO: 7);
[0032] (2) Screening by conventional histochemical staining method: the above polypeptides were diluted with PBS to 1000pM, 100pM, 10pM, 1pM and 0.1pM respectively, and each sample (polypeptide dilution) was compared with human hepatocellular carcinoma paraffin tissue sections (after routine pretreatment). Treatment) incubate at room temperature for 30 minutes; wash with PBS buffer (phosphate buffer); then add diluted HRP-labeled SA (SA-HRP) and incubate at room temperature for 15 minutes; wash with PBS buffer; Aniline) solution was incubated for 15 minutes; washed ...
Embodiment 2
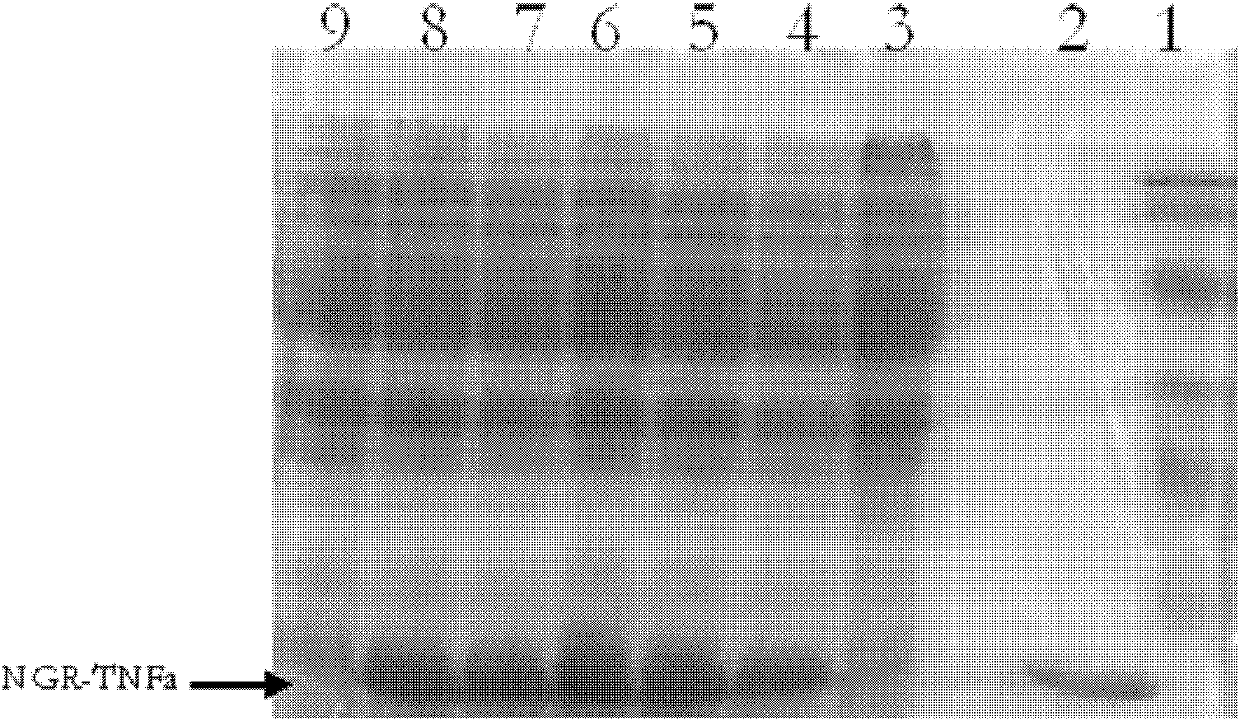
[0040] Expression and detection of NGR-TNFα fusion protein in Escherichia coli:
[0041] (1) Construction of recombinant expression vector:
[0042] (2) Carry out according to conventional molecular cloning techniques. It consists of the CD13 targeting peptide NGR and TNFα. The full length of the NGR-TNFα gene provided by the present invention is 507, encoding 168 amino acids, and its gene sequence is shown in SEQ ID NO:2. The corresponding amino acid sequence is shown in SEQ ID NO:3.
[0043] (3) Induced expression of the NGR-TNFα fusion protein in Escherichia coli:
[0044] (4) A. chemical synthesis method to obtain the target gene;
[0045] B. Construction of recombinant expression vector: the expression plasmid pBV220 was double-digested with restriction endonucleases (same as the restriction site of the primer), and then ligated into vector pBV220 under the action of ligase after digestion. The connection reaction system is:
[0046]
[0047] C. Obtain the expressi...
Embodiment 3
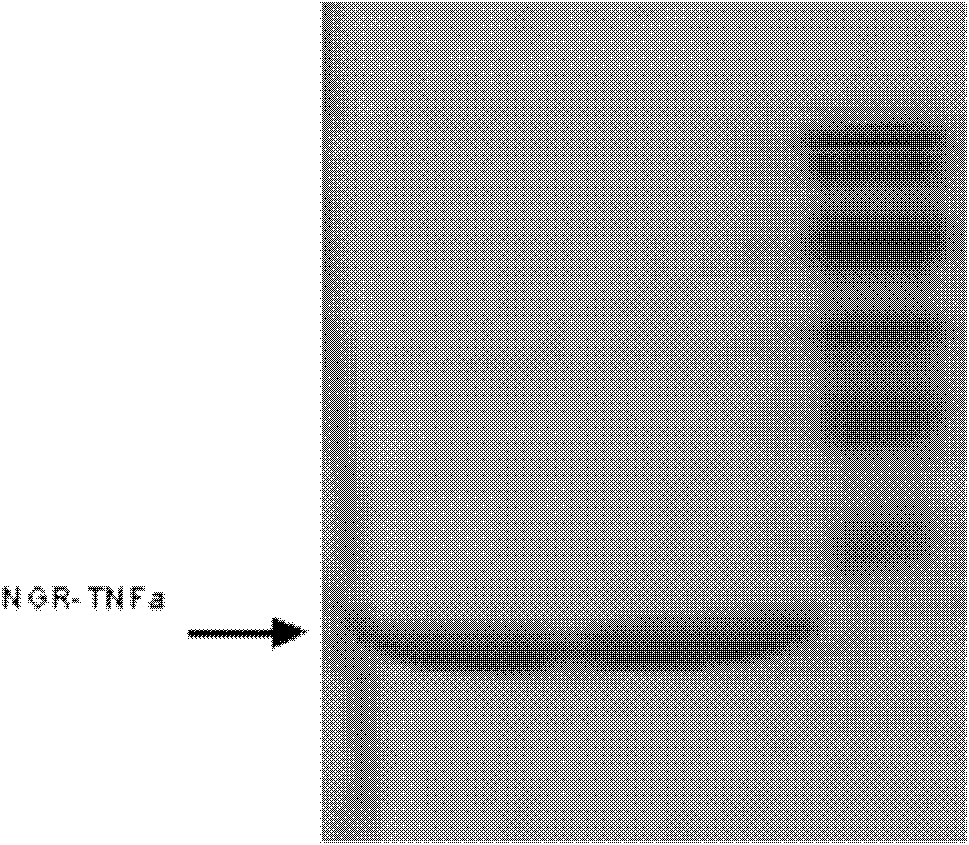
[0051] Purification of the NGR-TNFα fusion protein:
[0052] According to the conventional method, the process is: cation exchange column chromatography; ammonium sulfate salting out; hydrophobic column chromatography; anion exchange column chromatography. The brief experimental steps are as follows:
[0053] A. Induced engineering bacteria expressing NGR-TNFα;
[0054] B. Ultrasonic crush the engineering bacteria obtained in step A in an ice bath, centrifuge, and take the supernatant after it precipitates;
[0055] C. Dilute the supernatant obtained in step B 10 times with 10 mMPB (pH 6.0), and purify the resulting dilution by cation exchange column chromatography;
[0056] D. Add the purified product obtained in step C to 50% ammonium sulfate for precipitation (4°C, overnight);
[0057] E. Centrifuge the solution obtained in step D, and take the precipitate, which is dissolved in 2M ammonium sulfate;
[0058] F. the ammonium sulfate solution obtained in step E is purifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com