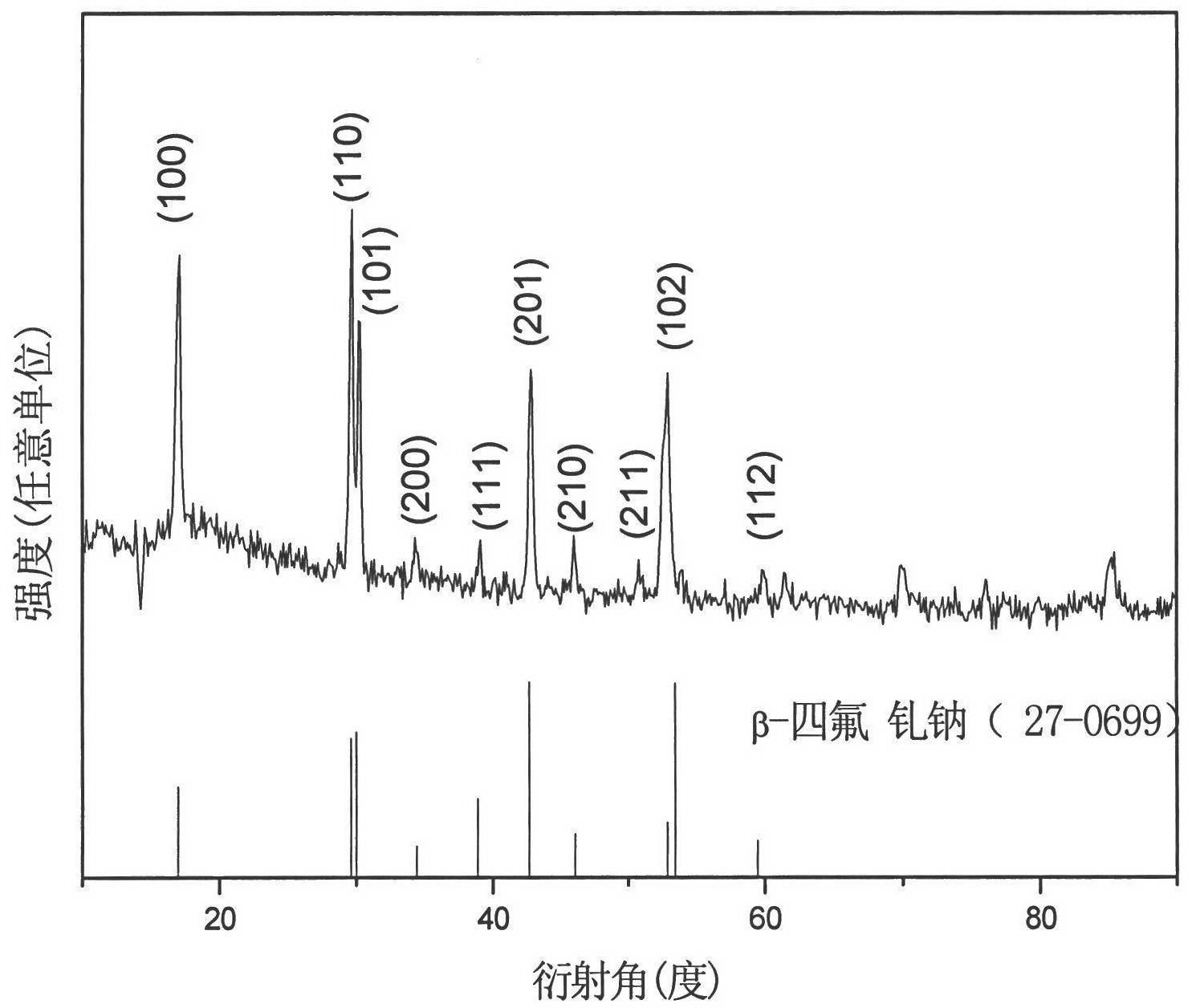
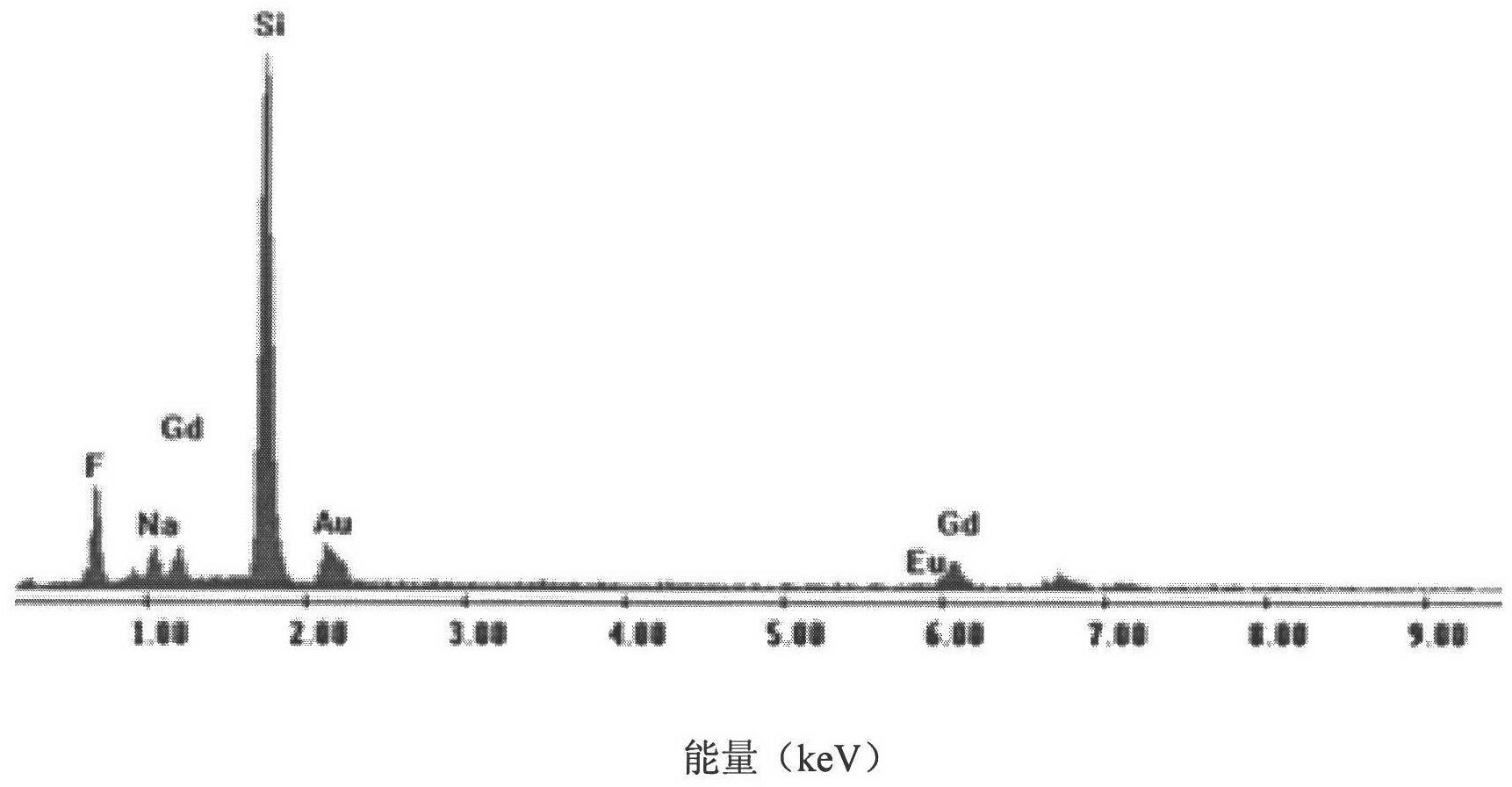
Europium ion-doped sodium gadolinium tetrafluoride luminescent nanorod and preparation method thereof
A technology of nano-rods and rare-earth ions is applied in the field of preparation of rare-earth doped light-emitting materials, and achieves the effect of strong practicability and wide application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Weigh 430mg Gd 2 o 3 and 22mg Eu 2 o 3 In a beaker, add 5mL of dilute nitric acid (the volume ratio of nitric acid and deionized water is 1:1) to dissolve, add 21mL of deionized water after cooling to form a rare earth ion solution; weigh 1.44g of sodium lauryl sulfate and add it to 9mL of deionized In water, after being completely dissolved, mix the two solutions, and stir with a magnetic stirrer at room temperature for 30 minutes to obtain a mixed solution of rare earth ions and sodium dodecyl sulfate; weigh 1.11g of ammonium fluoride and dissolve it in 5mL of deionized water to obtain fluoride Ammonium fluoride solution: Add the ammonium fluoride solution dropwise to the mixed solution of rare earth ions and sodium lauryl sulfate, continue stirring at room temperature for 30 minutes to obtain a mixed solution, transfer it to a 50mL reaction kettle, and react at 180°C After 24 hours, the reactor was taken out, naturally cooled to room temperature, cen...
Embodiment 2
[0020] Embodiment 2: Weigh 362.5mg Gd 2 o 3 and 70.4mg Eu 2 o 3 In a beaker, add 5mL of dilute nitric acid (the volume ratio of nitric acid and deionized water is 1:1) to dissolve, add 21mL of deionized water after cooling to form a rare earth ion solution; weigh 1.80g of sodium lauryl sulfate and add it to 9mL of deionized In water, after being completely dissolved, mix the two solutions, and stir with a magnetic stirrer at room temperature for 30 minutes to obtain a mixed solution of rare earth ions and sodium dodecyl sulfate; weigh 1.26g of sodium fluoride and dissolve it in 5mL of deionized water to obtain fluoride Sodium fluoride solution: Add sodium fluoride solution dropwise to the mixed solution of rare earth ions and sodium dodecyl sulfate, continue stirring at room temperature for 30 minutes to obtain a mixed solution, transfer it to a 50mL reaction kettle, and react at 180°C After 24 hours, the reactor was taken out, naturally cooled to room temperature, centrifu...
Embodiment 3
[0021] Embodiment 3: take by weighing 442mgY 2 o 3 and 11 mg Eu 2 o 3 In a beaker, add 5mL of dilute nitric acid (the volume ratio of nitric acid and deionized water is 1:1) to dissolve, add 21mL of deionized water after cooling to form a rare earth ion solution; weigh 1.08g of sodium lauryl sulfate and add it to 9mL of deionized After being completely dissolved in water, the two solutions were mixed, and stirred with a magnetic stirrer at room temperature for 30 minutes to obtain a mixed solution of rare earth ions and sodium lauryl sulfate. Weigh 1.26g of sodium fluoride and dissolve it in 5mL of deionized water to obtain a sodium fluoride solution; add the sodium fluoride solution dropwise to the mixed solution of rare earth ions and sodium lauryl sulfate, and continue stirring at room temperature for 30 minutes to obtain a mixed solution. solution, transferred it to a 50mL reaction kettle, reacted at 180°C for 36h, took out the reaction kettle, cooled to room temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com