Method for determining minimal inhibitory concentration of drug
A technology of minimum inhibitory concentration and drug, applied in material excitation analysis, fluorescence/phosphorescence, etc., can solve the problem of long measurement time, and achieve the effect of low cost, good application prospect and market prospect, fast, accurate and simple.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
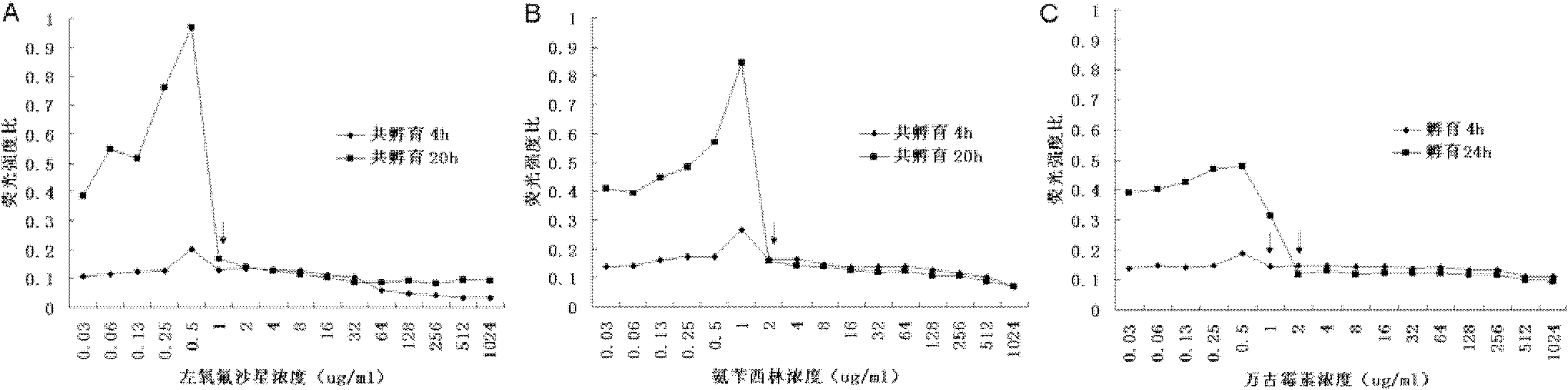
[0032] The rapid fluorescence method described in embodiment 1 detects the MIC value of ampicillin, levofloxacin, vancomycin to Enterococcus faecalis ATCC 29212
[0033] (1) Antibiotic preparation: refer to the MIC reference value of each antibiotic against Enterococcus provided by CLSI (2010 version), set the drug concentration gradient as 1024, 512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125 μg / ml. Antibacterial drugs of different concentrations after serial dilution were added to sterile 96-well polystyrene plates, 100 μl per well, and three replicate wells were made for each gradient. At the same time, negative control wells (70% ethanol treatment group) and no drug-treated wells were set as growth control (positive control).
[0034] (2) Preparation of bacterial suspension: Dilute the bacterial suspension of 0.5 McFarland turbidimetric standard 10 times with MH broth (Mueller-Hinton Broth, OXOID), and add it to each well containing different concentrations of dru...
Embodiment 2
[0042] (1) Antibiotic preparation: refer to the MIC reference value of each antibiotic against Enterococcus provided by CLSI (2010 version), set the drug concentration gradient as 512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125 μg / ml. Antibacterial drugs of different concentrations after serial dilution were added to sterile 96-well polystyrene plates, 100 μl per well, and three replicate wells were made for each gradient. At the same time, negative control wells (70% ethanol treatment group) and no drug-treated wells were set as growth controls (positive control).
[0043] (3) Preparation of bacterial suspension: Dilute the 0.5 McFarland turbidimetric standard bacterial suspension 10 times with MH broth (Mueller-Hinton Broth, OXOID), and add it to each well containing different concentrations of drugs, 100 μl / well, the final concentration is 2.5×10 6 CFU / ml. After sealing, put it in a normal air incubator at 34.5°C and incubate for 4 hours (the effect of vancomyci...
Embodiment 3
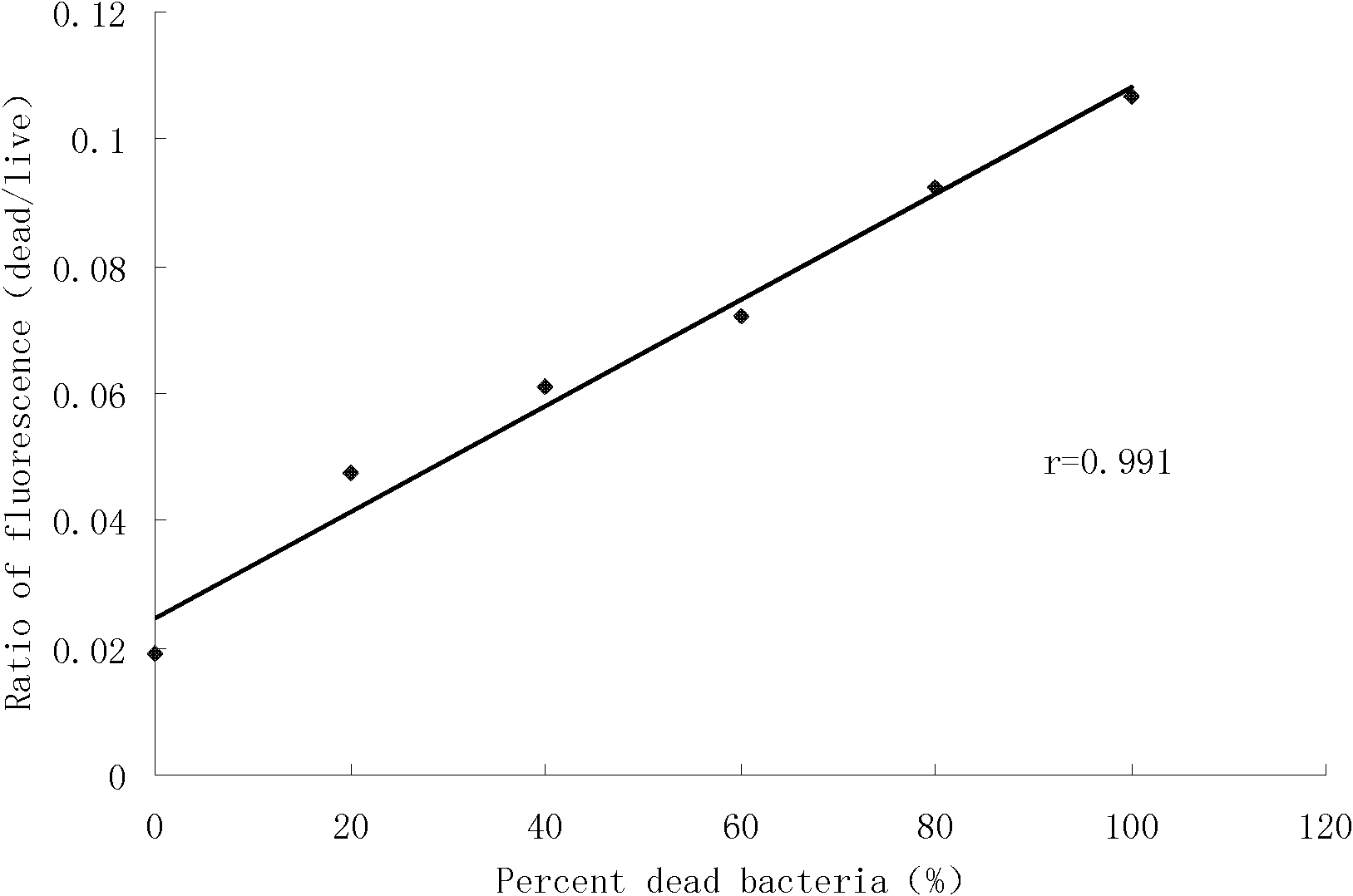
[0049] Example 3 The method of the present invention was applied to 90 strains of enterococcus clinically isolated, and compared with the MIC results obtained by the microdilution method performed in parallel, to verify the feasibility of the rapid fluorescence method for detecting MIC.
[0050] 1. Materials and methods
[0051] 1. Strains and Antibiotics
[0052] 1.1 Strains: Enterococcus faecalis standard strain ATCC 29212; 90 clinically isolated Enterococcus strains, 90 of which were identified as Enterococcus faecalis, of which 75 were Enterococcus faecalis and 15 were Enterococcus faecium.
[0053] 1.1 Antibiotics: Standard products of levofloxacin, ampicillin and vancomycin were purchased from China National Institutes for Food and Drug Control. The three drug standard products were made into stock solutions with sterile deionized water, aliquoted and stored at -70°C.
[0054] 2. Method
[0055] 2.1 Determination of MIC
[0056] In this study, the minimum inhibitory ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com