The synthetic preparation method of 2,6-dinitrobenzaldehyde
A technology of dinitrobenzene and dinitrotoluene, applied in the field of synthesis and preparation of 2,6-dinitrobenzaldehyde, can solve the problems of harsh reaction conditions, difficult industrial production, serious pollution, etc., and achieve easy operation , short cycle and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
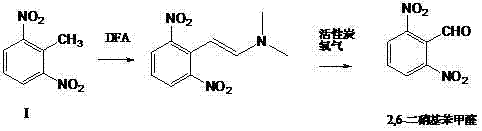
[0021] The preparation of embodiment 1 2,6-dinitrobenzaldehyde
[0022] Dissolve 2,6-dinitrotoluene (9.10g, 0.05mol) and DFA (35.70g, 0.3mol) in 90ml of organic solvent dioxane, heat at 100°C for 8 hours, and concentrate and evaporate to dryness after the reaction Then add dichloromethane, add 0.5g of activated carbon, pass oxygen at normal pressure and room temperature, filter after the oxidation reaction is completed, concentrate the filtrate to dryness, and recrystallize in ethanol to obtain 6.4g of 2,6-dinitrobenzaldehyde yellow solid , yield 65.3%. The resulting product Mp (melting point) 120-122°C.
Embodiment 2
[0023] Example 2 Preparation of 2,6-dinitrobenzaldehyde
[0024] 2,6-Dinitrotoluene (9.10 g, 0.05 mol) and DFA (35.70 g, 0.3 mol) were dissolved in 50 ml of organic solvent DMF, and heated at 100°C for 8 hours. After the reaction is completed, concentrate and evaporate to dryness, then add it to dichloromethane, add 0.5g of activated carbon, and pass through oxygen at normal pressure and room temperature, filter after the oxidation reaction is completed, concentrate the filtrate to dryness, and recrystallize in ethanol to obtain 2,6-dinitrate 6.2 g of benzaldehyde yellow solid, yield 63.2%. Mp 121-123°C.
Embodiment 3
[0025] Example 3 Preparation of 2,6-dinitrobenzaldehyde
[0026] 2,6-Dinitrotoluene (9.10 g, 0.05 mol) and DFA (35.70 g, 0.3 mol) were dissolved in 50 ml of organic solvent DMF, and heated at 100°C for 8 hours. After the reaction is completed, concentrate and evaporate to dryness, then add to methanol, add 0.5g of activated carbon, pass through oxygen at normal pressure and room temperature, filter after the oxidation reaction is completed, concentrate the filtrate to dryness, and recrystallize in ethanol to obtain 2,6-dinitrobenzene Formaldehyde yellow solid 6.5g, yield 66.3%. Mp 122-123°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com