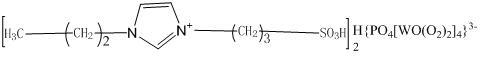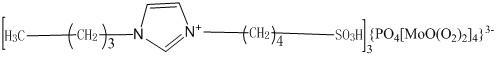Ionic liquid based on peroxyheteropolyacid and preparation method thereof
A technology of peroxopolyacid and ionic liquid, applied in organic chemistry and other directions, to achieve strong oxidizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] a) Add 100mL toluene and 75mL N-propylimidazole to a 250mL four-necked flask respectively, and slowly add 23g of 1,3-propane sultone dropwise into the four-necked flask at 60°C under the protection of nitrogen. After completion, the reaction was continued for 3 h to produce a white solid, cooled, filtered, washed with 50 mL of toluene and acetone, respectively, and dried in vacuum at 60° C. for 24 h to obtain an ionic liquid intermediate.
[0022] b) Take 2.88g of phosphotungstic acid and add it to a 100mL four-necked flask. At room temperature, slowly drop 10mL of 30% hydrogen peroxide into the four-necked flask. After the dripping, continue the reaction at 30°C for 1.5h , to obtain peroxopolyacid solution.
[0023] c) Get 0.55g of the ionic liquid intermediate prepared in a) and add it to a four-necked flask, and slowly add the peroxopolyacid solution prepared in b) dropwise to the four-necked flask at room temperature. After dripping, Continue to react at 60°C for 5...
example 2
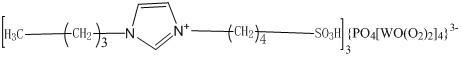
[0026] a) Add 100mL toluene and 75mL N-propylimidazole to a 250mL four-necked flask respectively, and slowly drop 25g of 1,4-butane sultone into the four-necked flask at 60°C under the protection of nitrogen. After completion, the reaction was continued for 3 h to produce a white solid, cooled, filtered, washed with 50 mL of toluene and acetone, respectively, and vacuum-dried at 60° C. for 24 h to obtain an ionic liquid intermediate.
[0027] b) Take 2.88g of phosphotungstic acid and add it to a 100mL four-necked flask. At room temperature, slowly drop 10mL of 30% hydrogen peroxide into the four-necked flask. After the dripping, continue the reaction at 30°C for 1.5h , to obtain peroxopolyacid solution.
[0028] c) Get 0.3g of the ionic liquid intermediate prepared in a) and add it to a four-necked flask, and slowly add the peroxopolyacid solution prepared in b) to the four-necked flask at room temperature. After the drop, Continue to react at 60°C for 5h, cool, filter, wash ...
example 3
[0031] a) Add 100mL toluene and 75mL N-octylimidazole to a 250mL four-necked flask respectively, and slowly add 25g of 1,4-butane sultone dropwise into the four-necked flask at 60°C under nitrogen protection. After completion, the reaction was continued for 3 h to produce a white solid, which was cooled and filtered. The white solid was washed with 50 mL of toluene and acetone respectively, and dried in vacuum at 60° C. for 24 h to obtain an ionic liquid intermediate.
[0032] b) Take 2.88g of phosphotungstic acid and add it to a 100mL four-necked flask. At room temperature, slowly drop 10mL of 30% hydrogen peroxide into the four-necked flask. After the dripping, continue the reaction at 30°C for 1.5h , to obtain peroxopolyacid solution.
[0033] c) Get 0.95g of the ionic liquid intermediate prepared in a) and add it to a four-necked flask, and slowly add the peroxopolyacid solution prepared in b) to the four-necked flask at room temperature. After the drop, Continue to react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com