Fluorescent quantitative pcr detection kit and non-diagnostic detection method for novel type A h1n1 virus
A detection method and fluorescence quantitative technology, which can be used in microorganism-based methods, microorganism determination/inspection, biochemical equipment and methods, etc. The effect of good performance and lower detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Detection of Primer Sensitivity
[0036] 1) Select the full-length gene fragment of novel influenza A (H1N1) influenza HA synthesized in vitro as a standard, and increase its viral RNA concentration from 10 -1 sequentially diluted to 10 -10 ;
[0037] 2) Negative control: normal throat swab RNA;
[0038] 3) The reaction system is prepared according to Table 3;
[0039] 4) Primer-probe combination: the primer-probe combination (YHA) designed by the present invention and the primer-probe combination (WHO-SWH1) published by the World Health Organization.
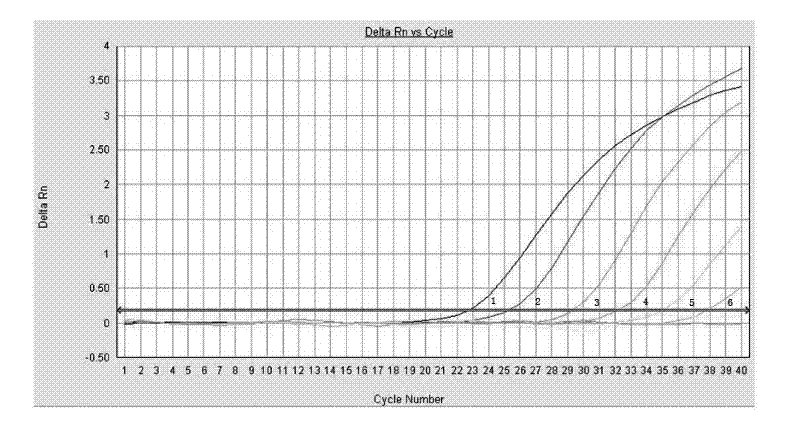
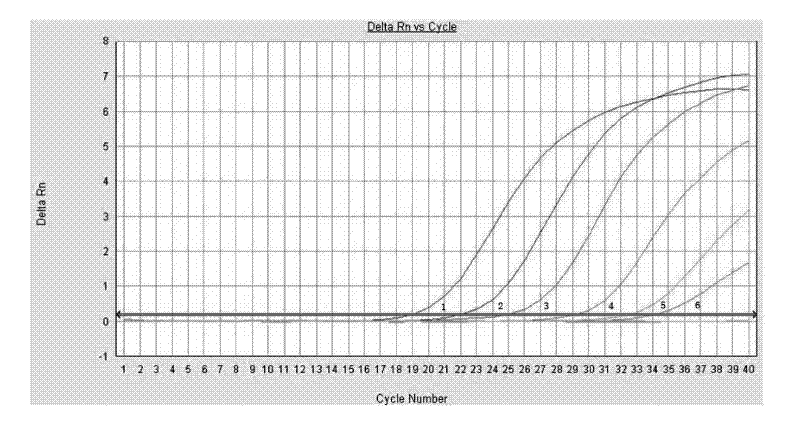
[0040] Test results such as figure 2 , 3 shown.
[0041] figure 2 The curves 1-6 in the middle are respectively the concentration dilution of the standard substance is 10 -4 -10 -10 The curve amplified with the primer-probe combination (WHO-SWH1) published by the World Health Organization. image 3 The curves 1-6 in the middle are the concentration dilution of the standard substance in order of 10 ...
Embodiment 2
[0045] Embodiment 2: Detection of primer characteristics
[0046]1) Non-specific detection of common seasonal influenza H1N1, swine influenza H1N1, avian influenza H5N1, H9N2;
[0047] 2) Positive control: HA fragment RNA of new type A H1N1 influenza virus;
[0048] 3) Negative control: Normal throat swab RNA;
[0049] 4) The reaction system was prepared according to Table 2.
[0050] See the test results Figure 4 and Table 5.
[0051] Figure 4 The results showed that only positive control HA RNA numbered 1 (10 -6 ) showed an amplification curve. The curves of the remaining samples were all below the baseline, and there was no amplification reaction.
[0052] Table 5 Human, poultry and swine influenza virus detection results
[0053]
[0054] The data in Table 5 shows that the primer probe combination designed by the present invention has a CT value of "-" for gene amplification of common seasonal influenza H1N1, swine influenza H1N1, avian influenza H5N1 and H9N2...
Embodiment 3
[0055] Embodiment 3: detection of virus culture
[0056] 1) Detect the virus culture and screen the new type A H1N1 influenza virus;
[0057] 2) Positive control: HA fragment RNA of new type A H1N1 influenza virus;
[0058] 3) Negative control: Normal throat swab RNA;
[0059] 4) The reaction system was prepared according to Table 3.
[0060] See the test results Figure 5 and Table 6.
[0061] Figure 5 Only one amplification curve of the positive control appeared, and the rest of the samples had no amplification. The reaction was negative.
[0062] Table 6 Virus culture test results
[0063]
[0064]
[0065] The results of the data in Table 6 show that the results of the detection of virus cultures were all negative.
[0066] Result analysis:
[0067] After the amplification reaction has completed 40 cycles, the sensitivity of the primer probe combination designed by the present invention detects that the viral RNA concentration dilution is 10 -9 Compared wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com