Application of high-purity baicalin or baicalein to preparation of inhaled asthma relieving medicament
A technology of baicalin and baicalein, applied in the application field of baicalin or baicalein in the preparation of inhaled anti-asthmatic drugs, to achieve the effect of alleviating bronchospasm, controlling acute attacks, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Inhaled asthma powder:
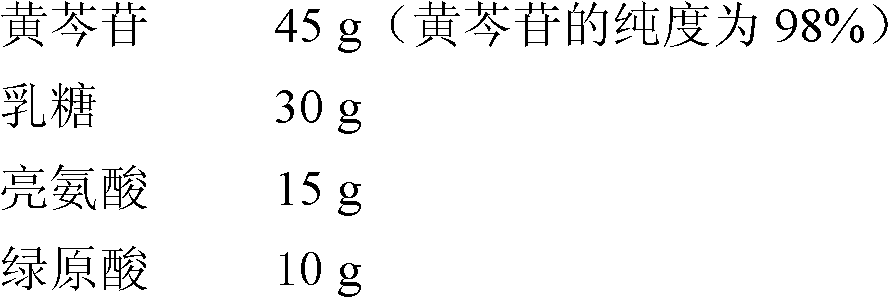
[0032] prescription
[0033]
[0034] Preparation method: Micronize baicalin, select lactose and leucine with a particle size of 0.5-5 μm as carriers, add an appropriate amount of 50% (v / v) ethanol aqueous solution to granulate, place in a rotary vibrating sieve, shake for 50 minutes, and dry That is, the particle size is 0.5-5 μm. After intermediate analysis and content determination, it is filled into capsules, and each capsule contains 30 mg of the main drug, and is used by means of a capsule-type propeller-type dry powder inhaler.
Embodiment 2
[0036] Inhaled asthma powder:
[0037] prescription
[0038] Baicalin 15g (the purity of baicalin is 91%)
[0039] Lactose 60g
[0040] Poloxamer 0.03g
[0041] Preparation method: After uniformly mixing the components in the above prescription, add an appropriate amount of 95% (v / v) ethanol aqueous solution to form a solution, micronize it by spray drying to make the average particle size reach 1-5 μm, and sieve through a 100-mesh sieve. Capsules. After intermediate analysis and content determination, it is filled into capsules and used by means of capsule-type propeller dry powder inhalers.
Embodiment 3
[0043] Inhaled asthma powder:
[0044] prescription:
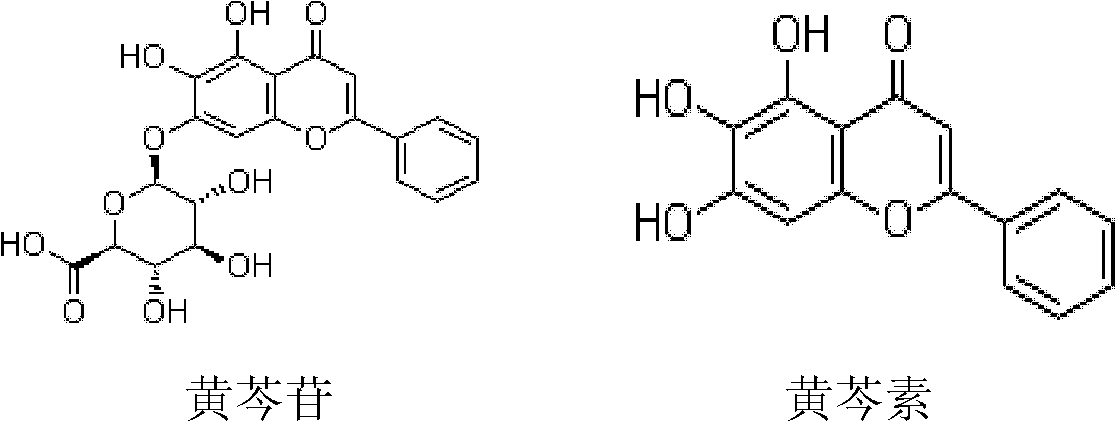
[0045] Baicalin 0.1g (the purity of baicalin is 98%)
[0046] Mannitol 98g
[0047] Preparation method: baicalin is spray-dried, and then nano-powder is prepared by high-pressure homogeneous method, with a particle size of 100-900nm; mannitol is made into a micropowder of about 150 μm, and baicalin nanopowder and mannitol are made into a micropowder of about 150 μm and mixed evenly. 100 mesh sieve, packed in capsules. After intermediate analysis and content determination, it is filled into capsules and used by means of capsule-type propeller dry powder inhalers.
[0048] Mannitol in this example can also be replaced by polyethylene glycol 6000 or gum arabic to prepare inhalation powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com