Fenofibrate acid salt, preparation method, pharmaceutical composition and application
A technology for fenofibrate acid and medicine, applied in the field of medicine, can solve the problems of low absorption and utilization rate, complicated process, insoluble fenofibrate in water, etc., and achieve the effects of improved bioavailability and easy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
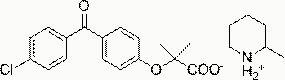
[0031] Fenofibric acid piperidine salt, its structural formula is:
[0032] .
[0033] The preparation method of fenofibric acid piperidine salt: dissolve 3.18g fenofibrate acid (10 mmol) in 20ml ethanol, add 0.8g piperidine (10mmol), heat and reflux for 0.5 hours, cool to room temperature, and gradually precipitate The white precipitate was filtered, collected, washed with ethanol, and dried in vacuo to obtain 3.18 g of fenofibric acid piperidine salt as a white solid, with a yield of 80%. Melting point 148-149°C, NMR analysis data 1 H NMR (400MHz, d 6 -DMSO): δ7.68-7.61 (m, 6H), 6.90 (bs, 2H), 2.89 (bs,4H ), 1.58-1.47 (m, 12H).
Embodiment 2
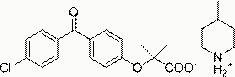
[0035] Fenofibric acid 2-methylpiperidinium salt, its structural formula is:
[0036] .
[0037] The preparation method of fenofibric acid 2-methylpiperidine salt: dissolve 3.18g fenofibric acid (10 mmol) in 20ml ethanol, add 0.94g 2-methylpiperidine (10mmol), heat and reflux for 0.5 Hours, cooled to room temperature, a white precipitate gradually precipitated, filtered, collected and washed with ethanol, dried in vacuo to obtain 3.05 g of fenofibric acid 2-methylpiperidine salt white solid, yield 74%. Melting point 135-136°C, nuclear magnetic analysis data 1 H NMR (400MHz, d 6 -DMSO): δ7.69-7.58 (m, 6H), 6.91 (d, J=8.8Hz, 2H), 3.10 (d, J=12.4Hz, 1H), 2.95-2.91 (m, 1H), 2.73- 2.66 (m, 1H), 1.68-1.64 (m, 3H), 1.59-1.51 (m, 7H), 1.41-1.27 (m, 2H), 1.13 (d, J=6.4Hz, 3H).
Embodiment 3
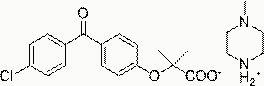
[0039] Fenofibric acid 3-methylpiperidinium salt, its structural formula is:
[0040] .
[0041] The preparation method of fenofibric acid 3-methylpiperidine salt: dissolve 3.18g fenofibric acid (10 mmol) in 20ml ethanol, add 0.94g 3-methylpiperidine (10mmol), heat and reflux for 0.5 Hours, cooled to room temperature, a white precipitate gradually precipitated, filtered, collected the precipitate and washed with ethanol, dried in vacuo to obtain 3.25 g of fenofibric acid 3-methylpiperidine salt as a white solid, with a yield of 79%. Melting point 131°C, nuclear magnetic analysis data1 H NMR (400MHz, d 6 -DMSO): δ 7.69-7.58 (m, 6H), 6.90 (d, J=8.8Hz, 2H), 3.08 (d, J=12Hz, 1H), 3.01 (d, J=10Hz, 1H), 2.63- 2.56 (m, 1H), 2.31 (t, J=12Hz, 1H), 1.69-1.63 (m, 4H), 1.48 (s, 6H), 0.81 (d, J=6.4Hz, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com